Abstract
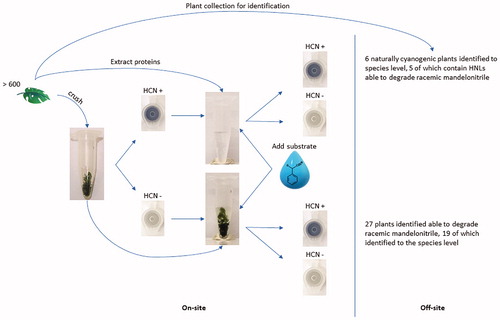
Hydroxynitrile lyases (HNLs) are sought-after, stereo-selective biocatalysts used in the agrochemical, pharmaceutical and fine chemical industries to produce cyanohydrin enantiomers. There are several approaches for the discovery of HNLs, most of which are methodologically demanding and not suitable for high-throughput. Bioprospecting studies to date have also been constrained/limited to commercialised plants or botanical gardens, leaving a vast majority of plant species untested for HNL activity or cyanogenesis. To increase the rate of discovery of HCN liberating plants, we devised a Feigl-Anger microfuge tube that is portable and capable of high throughput detection of naturally cyanogenic plants. A workflow suitable for detecting plant candidates containing extractable, novel HNLs was subsequently applied. In this study, we screened over 600 plants for cyanogenic activity as well as the ability to degrade racemic mandelonitrile. We detected 33 plants able to degrade racemic mandelonitrile, of which, 25 were identified to the species level. Six of these plants were found to be naturally cyanogenic. Protein extracts from 5 of the naturally cyanogenic plants retained the ability to degrade racemic mandelonitrile pointing to five yet undescribed enzymes in the species Achyranthes aspera, Davallia trichomonoides, Morus mesozygia, Polypodium aureum “Mandaianum”, and Thelypteris confluens. In contrast, although Acalypha glabrata was found to be naturally cyanogenic, the protein extract did not break down racemic mandelonitrile. Here, we used racemic mandelonitrile as substrate and detected enzymes with mandelonitrile lyase activity, however, any cyanohydrin could be used as part of the approach taken here to detect novel HNLs specific to the substrate utilised.
GRAPHICAL ABSTRACT
Introduction
Hydroxynitrile lyases (HNLs) are important biocatalysts to industries active in the production of agrochemicals, pharmaceuticals and fine chemicals (Bracco et al. Citation2016). The industrial significance of HNLs is derived from their ability to stereo-selectively catalyse the formation of a C-C bond, using an aldehyde or a ketone and hydrogen cyanide as substrates, to produce (R) or (S) cyanohydrins (Dadashipour and Asano Citation2011; Effenberger and Heid Citation1995; Padhi Citation2017; Wehtje et al. Citation1990). Cyanohydrins are sought after industrially due to the reactive hydroxy and nitrile functional groups which permit versatile conversions into valuable products within a few steps (Padhi Citation2017). HNLs can also catalyse the stereoselective production of β-nitro alcohols from aldehydes and MeNO2 (Fuhshuku and Asano Citation2011). There is a wide range of reviewed research areas involving the use of HNLs (Kassim and Rumbold Citation2014; Bracco et al. Citation2016) as well as methodologies for the discovery of new HNLs (Krammer et al. Citation2007; Dadashipour and Asano Citation2011; Padhi Citation2017). In recent years, however, the practice of bioprospecting for HNLs has been gaining increased attention (Asano et al. Citation2005; Takos et al. Citation2010; Kassim et al. Citation2014; Lanfranchi et al. Citation2015). Moreover, discovery of new HNLs has spurred cunning tactics for the identification of genes encoding for new HNLs in the absence of sequence homology (Lanfranchi et al. Citation2017).
The natural occurrence of HNLs is typically associated with cyanogenesis. Cyanogenesis, distinguished by the release of hydrogen cyanide, is believed to be triggered in response to herbivores, predators and infectious microorganisms (Jones Citation1998). This mechanism is predominantly found in plants, belonging to at least 90 plant families (Conn Citation1969; Jones Citation1998; Asano et al. Citation2005; Kassim et al. Citation2014), however, it has also been reported alongside HNLs in bacteria (Hajnal et al. Citation2013; Wiedner et al. Citation2014) and several species of millipedes from the Paradoxosomatidae and Xystodesmidae families (Dadashipour et al. Citation2015; Yamaguchi et al. Citation2018). It has become an attractive option to screen for HNLs from natural sources closely related to those already known to produce HNLs, especially considering that plants do not necessarily have to be cyanogenic to contain HNLs and vice versa (Andexer et al. Citation2007; Kassim et al. Citation2014; Yamaguchi et al. Citation2018). Additionally, some HNLs are known to have evolved convergently to perform the same function (Omelchenko et al. Citation2010; Lanfranchi et al. Citation2017). As such, approaches to screen for HNLs that are unbiased to any speciation, increases the chance of discovering unique HNLs with specific biochemical properties. Nevertheless, bioprospecting numerous samples is often limited by methods that are not very well adapted to be functional in the field. Bioprospecting is also encumbered by the need to store and transport samples to a laboratory for analysis in conditions that preserve enzymatic function. In addition, sequential laboratory work dedicated to the identification of new HNLs can often be very demanding, laborious and not always fruitful. This limits the throughput of screening for novel HNLs (Asano et al. Citation2005; Padhi Citation2017). In such cases, it is desirable to screen for HNLs in the field in high throughput immediately after sample collection to maintain sample integrity. The approach of this study was to screen for cyanogenic plants irrespective of plant family. A simple and robust Feigl-Anger microfuge tube with a colour developing Feigl-Anger reagent in the cap allows for the in-field identification of cyanogenic sources. In this study we targeted plants. The portability of the microfuge tube allows for screening for HNL activity by adding selected cyanogenic substrate and buffer to samples on site. The substrate added could include a cyanohydrin of interest that is difficult to biotransform. The quick identification of cyanogenic plants and HNLs reduces the number of samples to collect, to only those that test positive. Thereafter, downstream laboratory testing can be focussed on testing samples with definite HNL activity.
Materials and methods
In-field testing for cyanogenic plants and mandelonitrile lyases
During the month of October, plant material from the University of the Witwatersrand (Braamfontein, Gauteng); Fernhaven (Pretoria, Gauteng) and iSimangaliso Wetland Park (St Lucia, KwaZulu-Natal) in South Africa were screened for HNL activity using an adapted Feigl-Anger test. A Feigl-Anger microfuge tube that can detect gaseous hydrogen cyanide from any source was developed. The cyanide detection tubes were prepared by adding 35 µL of Feigl-Anger solution to the centre of the cap of 1.5 mL or 2 mL microfuge tubes and allowed to dry under a fume hood. The Feigl-Anger solution was prepared by gradually mixing an equivalent volume of 1% 4,4′-methylenebis (N,N-dimethylaniline) with 1% copper(II)ethylacetoacetate dissolved in chloroform as described by (Takos et al. Citation2010) based on the Feigl-Anger cyanide test paper (Feigl and Anger Citation1966). The dry reagent forms a blue salt when the tetrabase methylenebis (N,N-dimethylaniline) is oxidised in the presence of gaseous hydrocyanic acid (Feigl and Anger Citation1966). Noteworthy, the cyanide detection tubes were prepared, stored in a dark at room temperature and used in the field within a week.
Sample sites were selected based on the abundance of visible flora. Samples were numerically tagged for tracking. Small portions of leaf tissue from 600 different species were collected, mechanically disrupted and placed inside the Feigl-Anger cyanide detection tubes. The tubes were closed, stored in dark containers and visually inspected at time intervals. Cyanogenic activity can be noted within 2–3 minutes, although, tubes were monitored for a maximum of 30 minutes. The formation of a blue spot on the cap of the Feigl-Anger microfuge tube indicated the release of gaseous hydrogen cyanide and, hence, indicated that the tissue is naturally cyanogenic.
After monitoring for the natural occurrence of cyanogenesis, the disrupted tissue in each Feigl-Anger microfuge tube was submerged in 100 µL of 10 mM racemic mandelonitrile prepared in 100 mM citrate buffer pH 4.5. This, in turn, allowed for the detection of mandelonitrile lyase activity in non-cyanogenic plants. However, to prevent the false positive detection due to chemical hydrolysis of mandelonitrile, the reaction was monitored for a maximum period of 5 minutes.
Cyanogenic specimens were tracked by numeric tag and collected in ample quantity for further analyses. To test for lyase activity, protein was extracted from plants exhibiting natural cyanogenesis using the P-PER Kit (ThermoFisher Scientific®). In brief, approximately 80 mg of plant tissue was macerated in polypropylene bags containing 1 mL of the P-PER working solution supplemented with 10 mM DTT (Sigma-Aldrich) and cOmpleteTM ULTRA protease inhibitor tablets (Roche). The crushed material in solution was then centrifuged at 5,000 x g for 5 minutes at room temperature. The lower aqueous phase containing extracted proteins (9–20 mg/mL) were desalted using the Zebra Spin 7 K MWCO desalting columns (ThermoFisher Scientific®). The desalted protein extracts (4–18 mg/mL) were tested again for mandelonitrile lyase activity using the Feigl-Anger microfuge tube. Protein extracts were routinely quantified using the Qubit fluorometer 2.0 (Life Technologies).
Plant identification
Plant specimens found to be cyanogenic as well as those that showed mandelonitrile lyase activity where photographed and the GPS coordinates of their point of origin were recorded. Thereafter, plant specimens were collected, pressed between newspaper sheets changed daily for drying, and separated by corrugated cardboard to allow ventilation. The plant press setup was tightened with straps and stored at room temperature in a well-ventilated room. The preservation process was adapted from (Victor et al. Citation2004). Once specimens were dry, they were glued to white paper and microwaved for 75 seconds to prevent insect damage. Identification was done at the South African national biodiversity institute (SANBI) in the national herbarium at the Pretoria National Botanical Gardens.
Results
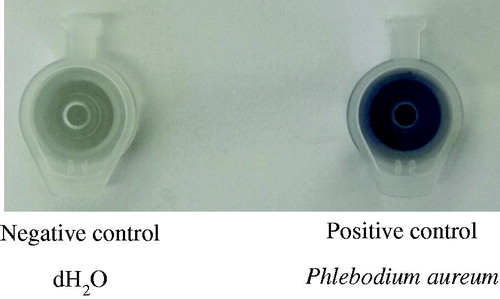
The Feigl-Anger microfuge tube cap turns blue in the presence of cyanide gas (). Crushed plant material inside the tube releases gaseous hydrocyanic acid, oxidising the tetra base inside the cap which then turns blue. Phlebodium aureum is a known producer of HNLs and cyanide (Wajant et al. Citation1995). Therefore, P. aureum (obtained from Fernhaven, Pretoria, South Africa) was mechanically crushed, enclosed within the Feigl-Anger microfuge tube and used as a positive control. In contrast, dH2O was used as a negative control ().
Figure 1. Controls for the ability of the Feigl-Anger microfuge tube to qualitatively detect the release of cyanide gas. As a negative control dH2O was used. As a positive control, crushed Phlebodium aureum leaf tissue was closed inside the Feigl-Anger microfuge tube.
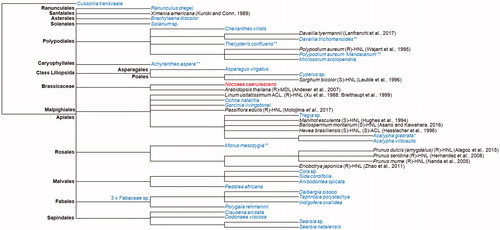
From over 600 plant species screened, we have identified 33 plants across 13 orders that can catalyse the reverse cyanohydrin reaction to form hydrocyanic acid and benzaldehyde from mandelonitrile. After collection, 25 of the plants were identified at the species level, 5 at the genus level and 3 were identified to be part of the Fabaceae family. Six of the specimens were found to be naturally cyanogenic. Namely, Acalypha glabrata, Achyranthes aspera, Davallia trichomonoides, Morus mesozygia, Polypodium aureum “Mandaianum” and Thelypteris confluens. Protein extracts from those plants were incubated with racemic mandelonitrile to verify the ability to catalyse the disintegration of mandelonitrile. This eliminates false positives generated as a result of an unknown cyanide containing substrate within the plant tissue. From the six cyanogenic plants, only protein extract from A. glabrata did not retain the enzymatic ability to degrade racemic mandelonitrile following protein extraction and desalting. The other protein extracts contained proteins with the ability to catalyse a lyase reaction. The species identified in this study are named in the unrooted common tree dendrogram alongside the species from which an HNL has been characterised and/or a sequence is available on the UniProt database (UniProt Citation2019) ().
Figure 2. Taxonomic common tree dendrogram of the plants identified in this study to have the ability to degrade racemic mandelonitrile (grey) and plants that have an HNL sequenceAQ11 available on the UniProt database and/or have HNLs characterised in literature (black). The UniProt sequence for a possible HNL in Noccaea caerulescens bares homology to HNLs, however, there is no literature indicating cyanogenic ability of the plant. * as a superscript indicates that the plant is naturally cyanogenic; ** as a superscript indicates that the proteins extracted with the P-PER Kit from the naturally cyanogenic plants retained MDL activity in the presence of racemic mandelonitrile. Branch nodes representing the order are labelled in black and bolded.
Discussion
The Feigl-Anger microfuge tube is easily prepared, portable and simple to use. It is advantageous for unguided and untargeted in-field screening of cyanogenic sources and HNLs. The in-field testing of the freshest possible material prevents the extensive degradation of enzymes caused by time, incompatible storage and transport conditions or lack of storage facilities for a large number of samples. It also prevents the need to collect unnecessarily high amounts of plant tissue samples. Thus, permitting for selective collection of lead candidates for HNLs Thereafter, plant samples able to catalyse the breakdown of added cyanohydrin substrates into hydrogen cyanide and aldehyde/ketone can be studied in more depth for substrate compatibility, promiscuity and ultimately for hydroxynitrile lyase sequences. The Feigl-Anger microfuge tube can be used to bioprospect in the field with any cyanohydrin for which a biocatalyst is wanted.
Using the Feigl-Anger microfuge tube in this study, we have identified 25 plant species that -although not cyanogenic – were able to degrade racemic mandelonitrile which can be investigated further for the presence of mandelonitrile lyases. Although, HNLs may possibly still be present in specimens that do not have substrate available (Andexer et al. Citation2007; Kassim et al. Citation2014; Yamaguchi et al. Citation2018), it may also be useful to test those plants for natural cyanogenic ability in other seasons, climates, as well as, tissue types and maturity to increase chances of extracting enough amounts of HNLs for downstream characterisation. It remains uncertain if species identified as non-cyanogenic (but able to degrade racemic mandelonitrile) in this study are, in fact, completely devoid of cyanogenic activity since this mechanism has been found to be influenced by seasonal changes, climate, tissue type and tissue maturity (Gleadow and Woodrow Citation2000).
Four of 5 species identified in this study to be naturally cyanogenic and able to degrade racemic mandelonitrile after protein extraction provide promising candidates for the identification of HNLs with similarity to known HNLs (). Furthermore, Achyranthes aspera is the only plant known to date to exhibit HNL activity in the order Caryophyllales which opens questions regarding the homology and biochemistry of the HNL within the order. It is likely to harbour a distinct HNL with yet unexplored biochemical properties. Acalypha glabrata, in contrast, was found to be naturally cyanogenic but not able to degrade racemic mandelonitrile after protein extraction and desalting, possibly due to incompatibilities with kit components or inherently low stability. A. glabrata is part of the same order as Linum usitatissimum and Hevea brasiliensis, both of which harbour acetone cyanohydrin lyase activity (Hasslacher et al. Citation1996; Xu et al. Citation1988). This may suggest that A. glabrata which is naturally cyanogenic, may prefer acetone cyanohydrin or another structurally distinct substrate to mandelonitrile. Just as well, the A. glabrata HNL may be FAD-dependent and may have lost the co-factor compromising the structure and function of the enzyme (Dreveny et al. Citation2009) as a result of the protein extraction method carried out here. Nevertheless, it is worth investigating A. glabrata further as a possible acetone cyanohydrin lyase candidate.
As part of this study we have devised a Feigl-Anger microfuge tube capable of high-throughput sampling for cyanogenic sources in the field. With this robust and simple tool, we have identified 25 plant species able to degrade racemic mandelonitrile. Achyranthes aspera, Davallia trichomonoides, Morus mesozygia, Polypodium aureum “Mandaianum”, and Thelypteris confluens are naturally cyanogenic and enzymatic extracts from those retained MDL activity. They are potential candidates for further characterisation. Acalypha glabrata is naturally cyanogenic, however, enzymatic extracts from the tissue did not retain MDL activity, possibly due to diverging substrate specificities, which makes this candidate a particularly interesting source of a novel HNL.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Graphical Abstract
Download TIFF Image (192.2 KB)Acknowledgements
We would like to thank Dr. Chris Mayburg, the owner of Fernhaven (Pretoria), for kindly granting us access to screen the plants under his care as part of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andexer J, von Langermann J, Mell A, Bocola M, Kragl U, Eggert T, Pohl M. 2007. An R-Selective hydroxynitrile lyase from arabidopsis thaliana with an α/β-Hydrolase fold. Angew Chem Int Ed. 46(45):8679–8681.
- Asano Y, Tamura K, Doi N, Ueatrongchit T, H-Kittikun A, Ohmiya T. 2005. Screening for new hydroxynitrilases from plants. Biosci Biotechnol Biochem. 69(12):2349–2357.
- Bracco P, Busch H, von Langermann J, Hanefeld U. 2016. Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases – a review. Org Biomol Chem. 14(27):6375–6389.
- Conn E.E. 1969. Cyanogenic glycosides. J Agric Food Chem. 17(3):519–526.
- Dadashipour M, Asano Y. 2011. Hydroxynitrile lyases: insights into biochemistry, discovery, and engineering. ACS Catal. 1(9):1121–1149.
- Dadashipour M, Ishida Y, Yamamoto K, Asano Y. 2015. Discovery and molecular and biocatalytic properties of hydroxynitrile lyase from an invasive millipede, Chamberlinius hualienensis. Proc Natl Acad Sci USA. 112(34):10605–10610.
- Dreveny I, Andryushkova A.S, Glieder A, Gruber K, Kratky C. 2009. Substrate binding in the FAD-dependent hydroxynitrile lyase from almond provides insight into the mechanism of cyanohydrin formation and explains the absence of dehydrogenation activity. Biochemistry. 48(15):3370–3377.
- Effenberger F, Heid S. 1995. (R)-Oxynitrilase catalyzed synthesis of (R)-ketone cyanohydrins. Tetrahedron Asymmetry. 6(12):2945–2952.
- Feigl F, Anger V. 1966. Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst. 91(1081):282–284.
- Fuhshuku K, Asano Y. 2011. Synthesis of (R)-β-nitro alcohols catalyzed by R-selective hydroxynitrile lyase from Arabidopsis thaliana in the aqueous–organic biphasic system. J Biotechnol. 153(3–4):153–159.
- Gleadow R.M, Woodrow I.E. 2000. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx. Tree Physiol. 20(9):591–598.
- Hajnal I, Łyskowski A, Hanefeld U, Gruber K, Schwab H, Steiner K. 2013. Biochemical and structural characterization of a novel bacterial manganese-dependent hydroxynitrile lyase. Febs J. 280(22):5815–5828.
- Hasslacher M, Schall M, Hayn M, Griengl H, Kohlwein S.D, Schwab H. 1996. Molecular Cloning of the Full-length cDNA of (S)-Hydroxynitrile Lyase from Hevea brasiliensis functional expression in Escherichia coli and Saccharomyces cerevisiae and identification of an active site residue. J Biol Chem. 271(10):5884–5891.
- Jones D.A. 1998. Why are so many food plants cyanogenic? Phytochemistry. 47(2):155–162.
- Kassim M.A, Rumbold K. 2014. HCN production and hydroxynitrile lyase: a natural activity in plants and a renewed biotechnological interest. Biotechnol Lett. 36(2):223–228.
- Kassim M.A, Sooklal S.A, Archer R, Rumbold K. 2014. Screening for hydroxynitrile lyase activity in non-commercialised plants. South Afr J Bot. 93:9–13.
- Krammer B, Rumbold K, Tschemmernegg M, Pöchlauer P, Schwab H. 2007. A novel screening assay for hydroxynitrile lyases suitable for high-throughput screening. J Biotechnol Enzyme Tech Biocatal. 129:151–161.
- Lanfranchi E, Köhler E.-M, Darnhofer B, Steiner K, Birner-Gruenberger R, Glieder A, Winkler M. 2015. Bioprospecting for hydroxynitrile lyases by blue native PAGE Coupled HCN Detection. CBIOT. 4(2):111–117.
- Lanfranchi E, Pavkov-Keller T, Koehler E.-M, Diepold M, Steiner K, Darnhofer B, Hartler J, Van Den Bergh T, Joosten H.-J, Gruber-Khadjawi M, Steiner K, Darnhofer B, Hartler J, Van Den Bergh T, Joosten HJ, Gruber-Khadjawi M, et al. 2017. Enzyme discovery beyond homology: a unique hydroxynitrile lyase in the Bet v1 superfamily. Sci Rep. 7:46738.
- Omelchenko M.V, Galperin M.Y, Wolf Y.I, Koonin E.V. 2010. Non-homologous isofunctional enzymes: a systematic analysis of alternative solutions in enzyme evolution. Biol Direct. 5(1):31.
- Padhi S.K. 2017. Modern approaches to discovering new hydroxynitrile lyases for biocatalysis. ChemBioChem. 18(2):152–160.
- Takos A, Lai D, Mikkelsen L, Hachem M.A, Shelton D, Motawia M.S, Olsen C.E, Wang T.L, Martin C, Rook F. 2010. Genetic screening identifies cyanogenesis-deficient mutants of Lotus japonicus and reveals enzymatic specificity in hydroxynitrile glucoside metabolism. Plant Cell. 22(5):1605–1619.
- UniProt. 2019. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47:D506–D515.
- Victor J.E, Koekemoer M, Fish L, Smithies S.J, Mössmer M. 2004. Herbarium essentials: the Southern African herbarium user manual (Article; Article/Report). Pretoria, South Africa: Southern African Botanical Diversity Network, National Botanical Institute.
- Wajant H, Forster S, Selmar D, Effenberger F, Pfizenmaier K. 1995. Purification and characterization of a Novel (R)-mandelonitrile lyase from the Fern Phlebodium aureum. Plant Physiol. 109(4):1231–1238.
- Wehtje E, Adlercreutz P, Mattiasson B. 1990. Formation of C-C bonds by mandelonitrile lyase in organic solvents. Biotechnol Bioeng. 36(1):39–46.
- Wiedner R, Gruber-Khadjawi M, Schwab H, Steiner K. 2014. Discovery of a novel (R)-selective bacterial hydroxynitrile lyase from Acidobacterium capsulatum. Comput Struct Biotechnol J. 10(16):58–62.
- Xu L.-L, Singh B.K, Conn E.E. 1988. Purification and characterization of acetone cyanohydrin lyase from Linum usitatissimum. Arch Biochem Biophys. 263(2):256–263.
- Yamaguchi T, Nuylert A, Ina A, Tanabe T, Asano Y. 2018. Hydroxynitrile lyases from cyanogenic millipedes: molecular cloning, heterologous expression, and whole-cell biocatalysis for the production of (R)-mandelonitrile. Sci Rep. 8(1):3051.
- Zhao G.-J, Yang Z.-Q, Guo Y.-H. 2011. Cloning and expression of hydroxynitrile lyase gene from Eriobotrya japonica in Pichia pastoris. J Biosci Bioeng. 112(4):321–325.
