Abstract
Objectives: To identify whether Pulsatilla saponin A (PsA), an active molecule extracted from Pulsatilla chinensis regel, can induce acute myeloid leukemia (AML) cells differentiate.
Methods: PsA was isolated from P. chinensis, and its effects of differentiation induction on both AML cell lines and the primary leukemia cells were investigated.
Results: Compared with the untreated control, PsA induced the differentiation of U937 cells, K562 cells and HL-60 cells, represented as the increased CD15+ cells in a dose- and time-dependent manner in all the three AML cell lines, after PsA treatment. As the same time, the cell morphology of these AML cells was changed correspondingly; the cytoplasm/nuclei ratio was increased, basophilic cytoplasm was decreased, and eccentric nucleus and granules were also observed. Also, the same effects of differentiation induction by PSA were confirmed in the primary leukemia cells. However, the specific MEK/ERK inhibitor U0126 effectively abrogated the differentiation induced by PsA in vitro.
Conclusions: PsA can modify the differentiation activity of AML cells, probably though the MEK/ERK signaling pathway.
Introduction
As a most common malignancy affecting adults, acute myeloid leukemia (AML) was characterized by the rapid expansion of malignant myeloid clone in the bone marrow, and the accumulation of hematopoietic stem cells arrested at different stages of development and abnormal proliferations.Citation1 Despite the toxic side effects and a number of predominant disadvantages, chemotherapy still was overwhelming traditional treatment strategy for AML, which mainly focused on removal or elimination of cancerous cells. However, the differentiation-inducing therapy for AML seems to be another very promising approach. Recently, the discovery of potent differentiation inducers and their potential use in AML treatment has become the hottest subject in the field of biology.
With its anti-inflammatory properties, Pulsatilla chinensis regel has been widely used to treat several infectious disease, for example, intestinal amebiasis and malaria etc.Citation2,Citation3 Recent studies have reported that a series of saponin-related compounds from P chinensis possessed antitumor activities.Citation3–Citation6 To date, at least 15 saponins were found in P chinensis, of which, Pulsatilla saponin A (PsA) has been reported to demonstrate its antitumor effect by inducing DNA damage and causing G2 arrest and apoptosis in cancer cells.Citation7
In the present study, we showed that PsA could induce the differentiation of AML cells. We also performed cellular and biochemical experiments to investigate the possible molecular mechanisms related to this effect of PsA.
Materials and methods
Cell culture
Human leukemia cell line K562 was cultured in IMDM medium supplemented with 20% FBS (Gibco, NY, USA), 100 mg/ml of streptomycin, 100 IU/ml of penicillin. Human leukemia monocyte lymphoma cell line U937 and human myeloblastic leukemia cell line HL-60 were cultured in RPMI1640 medium supplemented with 20% FBS (Gibco, NY,USA), 100 mg/ml of streptomycin, 100 IU/ml of penicillin. All cells were incubated under 5% CO2 humidified atmosphere at 37°C.
The primary mononuclear cells were isolated from fresh bone marrows of acute myeloblastic leukemia (AML) patients by Ficoll-Paque PLUS density gradient (Amersham Biosciences, CA, USA). The primary monocytes were cultured with IMDM medium plus 10% FBS, 100 mg/ml streptomycin and 100 IU/ml penicillin, in a humidified atmosphere with 5% CO2 at 37°C. All patient data were reviewed by at least two independent investigators. The AML classification was based on the French–American–British guidelines. The present study was approved by the ethic committees at local hospitals and was conducted in accordance with the Declaration of Helsinki.
Drug treatment
PsA was extracted as previously described,Citation7 and was dissolved in dimethyl sulfoxide (DMSO, Sigma Chemical Company, MO, USA) as a stock solution. In all experiments, the cells were treated with PsA in serum-free medium for at least 1 hour before the addition of FBS. In some cases, the K562 cells were pretreated with MEK/ERK inhibitor U0126 (Cell Signaling Technology, MA, USA) for 15–30 minutes, before the PsA treatment.
Differentiation assay
For observation of morphology changes, K562 cells were treated with PsA for 48 hours and then stained with Liu's stain, and observed under microscope. The percentage of differentiated cells was determined by microscopically quantifying at least 200 cells per experimental condition. All data were reviewed by at least two independent investigators.
Flow cytometry analysis
After exposed to PsA, K562 cells, U937 cells and HL-60 cells were isolated and stained with the phycoerythrin (PE)-conjugated monoclonal mouse antibody for human CD15 (cell surface maker of mature granulocyte lineage and monocytes lineage) and CD11 to detect CD15+ and CD11+ cell population. The PE-conjugated anti-mouse-IgG was applied as a control to determine the background fluorescence when performing the flow cytometry analysis (FACScan; Becton Dickinson).
Western blot assay
After drug treatment, cells were harvested and lysed in immunoprecipitation lysate buffer with protease inhibitors (Beyotime, China). The Western blot assay was performed as previously described.Citation7 In brief, the same amount of each protein samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting using antibodies against ERK and phosphorylated-44/42 ERK (Cell Signal Technology, MA, USA). Nitrocellulose membranes were then developed using electro chemiluminescent reagents (Denville Scientific, NJ, USA) and exposed to X-ray films.
Statistical analysis
Each experiment was performed at least three repetitions, and the data were expressed as mean ± standard deviation (SD). The significance of the differences between the two groups was determined using Student's t-test. P values <0.05 were considered statistically significant.
Results and discussion
Recent reports have suggested that sponin derivatives, such as Pulsatilla saponin D and Astragalus saponins, displays their antitumor activities.Citation5,Citation6,Citation8 Our previous data shown that PsA, an active compound extracted from P chinensis, is a novel antitumor agent capable of inducing DNA damage and causing G2 arrest and apoptosis among hepatocellular carcinoma and pancreatic cancer cells.Citation7 However, the effects of PsA on leukemia cells have never been reported. In the current study, we attempted to determine the effects and potential mechanisms of PsA on AML cells.
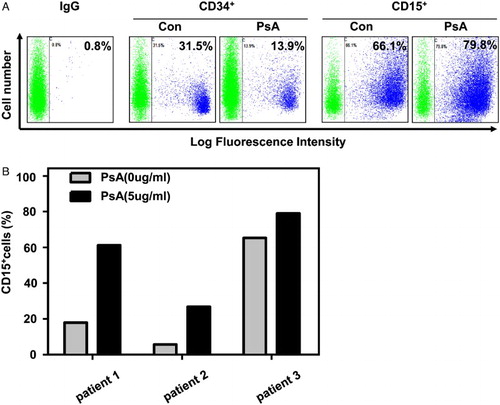
Firstly, PsA was purified from Pulsatilla chinensis regel as described before.Citation7 The molecule structure of PsA was shown in Fig. A. After incubation with different concentration of PsA for a certain time, all three AML cell lines K562, HL-60, and U937 were separately analyzed by FASC. In K562 cells, it was observed that PsA treatment could induce the differentiation in both a dose- and time-dependent manner (Fig. B and C), except for its apoptosis induction activity (supplementary Fig. ). The PsA induced differentiation was also observed in U937 and HL-60 cells in a dose-dependent manner (Fig. D). Similar as CD15 results, the CD11+ cells were also increased after treated with PsA in K562 (supplementary Fig. ). The differentiation induction activity of PsA was further confirmed by morphologic alteration of K562 cells. As shown in Fig. E and F, the increased cytoplasm/nuclei ratio, decreased basophilic cytoplasm, as while as eccentric nucleus and granules were presented in a certain proportion of K562 cells, after PsA treatment. In addition, primary AML cells isolated from patients were also utilized. Despite the low proliferation pattern of primary AML cells, the treatment with 5 µg/ml PsA for 72 hours still successfully eliminated CD34+ population, while induced more CD15+ cells (Fig. ). And the CD15+ cells were also increased in fresh cells from three de novo AML patients (supplementary Table 1) (Fig. B). Taken together, we demonstrated that PsA could effectively affect the differentiation of AML cell lines and primary AML cells in vitro, besides its apoptosis induction activity in cancer cells.
Figure 1 PsA induced differentiate in AML cell lines. (A) Chemical structure of PsA. The differentiation of K562 cells treated with the indicated concentrations of PsA for 48 hours (B) or 5 µg/ml PsA for the indicated time periods (C) was assessed. (D) AML cell lines U937, HL-60 were treated with the indicated concentrations of PsA for 48 hours. Differentiation was evaluated by measuring PE-conjugated CD15 membrane expression levels using flow cytometry. (E) The morphological changes in K562 cells treated with 5 µg/ml PsA for 48 hours. (F) Quantities the differentiated cells which were evaluated by the morphological changes after K562 cells treated with 5 µg/ml PsA for 48 hours Results represent the mean ± SD of at least three independent experiments for each cell line. Con: control, PsA: Pulsatilla saponin A, **P < 0.01, ***P < 0.001 compared to the untreated groups.
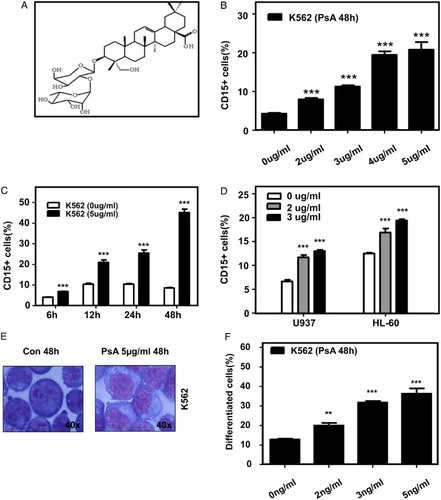
Figure 2 PsA induce differentiate in primary AML cells (A) CD34+ and CD15+ cells were detected after the primary AML cells treated with 5 μg/ml PsA for 72 hours. (B) CD15+ cells were detected in three de novo AML patients primary cells treated with 5 μg/ml PsA for 72 hours.
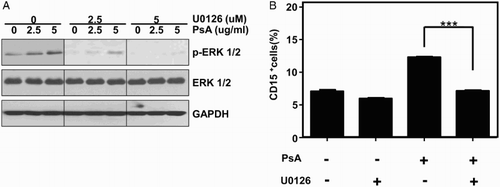
Next, we attempted to identify the possible molecular signaling events involved in granulocytic differentiation of AML cells under the influence of PsA. Based on the established function of MAPK in cell growth regulation, cell arrest and differentiation,Citation9–Citation11 recent studies have shown that the ERK activation is required for megakaryocytic differentiation.Citation12,Citation13 In this study, K562 cell line was chosen as a cell model to conduct the mechanism experiment, because it was regarded as one of the most classic human myelogenous leukemia cell line and has been extensively studied. The effect of PsA in combination with cell signaling pathway inhibitor on K562 cells was examined. After the treatment with 2.5 and 5 μg/ml of PsA at 37°C for 24 hours, we found the p-ERK protein expression was dose-dependently increased in the PsA treated cells (Fig. A). However, ERK inhibitor U0126 could eliminate the PsA induced ERK phosphorylation (Fig. A). As the same time, we noticed that U0126 significantly suppressed the PsA induced CD15+ subpopulation of K562, compared with PsA treated K562 cells (Fig. B). These results indicated that the MEK/ERK signaling pathway might also be essential for the PsA induced granulocytic differentiation of K562 cells.
Figure 3 ERK/MAPK signaling pathway might be involved in PsA induced differentiation in K562 cells (A) K562 cells were pretreated with either vehicle or U0126 for 30 minutes then exposed to PsA. Western blotting was performed to assess the expression of ERK and phosphorylated ERK. (B) CD15+ cells were detected with or without U0126 before exposed to PsA by FCAS. Results represent the mean ± SD of at least three independent experiments for each cell line. Con: control, PsA: Pulsatilla saponin A, **P < 0.01, ***P < 0.001 compared to the untreated groups.
In summary, PsA possessed differentiation induction activity in AML cell lines and primary AML cells, probably through ERK/MAPK signaling pathway. PsA might be a promising therapeutic reagent for AML as a novel class of differentiation inducer.
Disclaimer statements
Contributors X.Q. and R.Z. designed the experiments; T.W., R.Z., and F.T. performed experiments and analyzed data; J.N. collaborated for cell culture and L.D., M.Z. for FACS analysis; X.Q. and T.W. wrote the manuscript; and all authors critically reviewed and approved the final version of the manuscript.
Funding None.
Conflict of interest There are no conflicts-of-interest.
Ethical approval None.
Acknowledgments
The authors thank Dr. Jianquan Hou for support. This study was supported in part by grants from of the National Science Foundation of China (81170468), the Natural Scientific Foundation of Jiangsu Scientific Bureau (BK2011266), the Priority Academic Program Development of Jiangsu Higher Education Institutions, High-level talents in six industries of Jiangsu Province (WSN-066), 2012 Jiangsu Provincial Special Program of Medical Science (BL2012005), and Jiangsu Province's Key Medical Center (ZX201102).
References
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62. doi: 10.1056/NEJM199909303411407
- Cheng L, Zhang M, Zhang P, Song Z, Ma Z, Qu H. Silver complexation and tandem mass spectrometry for differentiation of triterpenoid saponins from the roots of Pulsatilla chinensis (Bunge) Regel. Rapid Commun Mass Spectrom. 2008;22(23):3783–90. doi: 10.1002/rcm.3801
- Xu QM, Shu Z, He WJ, Chen LY, Yang SL, Yang G, et al. Antitumor activity of Pulsatilla chinensis (Bunge) Regel saponins in human liver tumor 7402 cells in vitro and in vivo. Phytomedicine. 2012;19(3–4):293–300. doi: 10.1016/j.phymed.2011.08.066
- Shi BJ, Li Q, Zhang XQ, Ying W, Cai YW, Sheng YX. Triterpene glycosides from the aerial parts of Pulsatilla chinensis. Yao Xue Xue Bao. 2007;42(8):862–6.
- Kim Y, Bang SC, Lee JH, Ahn BZ. Pulsatilla saponin D: the antitumor principle from Pulsatilla koreana. Arch Pharm Res. 2004;27(9):915–8. doi: 10.1007/BF02975843
- Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28(6):1347–55. doi: 10.1093/carcin/bgl238
- Liu Q, Chen W, Jiao Y, Hou J, Wu Q, Liu Y, et al. Pulsatilla saponin A, an active molecule from Pulsatilla chinensis, induces cancer cell death and inhibits tumor growth in mouse xenograft models. J Surg Res. 2014;188(2):387–395. doi: 10.1016/j.jss.2014.01.026
- Son MK, Jung KH, Hong SW, Lee HS, Zheng HM, Choi MJ, et al. SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway. Food Chem. 2013;136(1):26–33. doi: 10.1016/j.foodchem.2012.07.096
- Rojnuckarin P, Drachman JG, Kashunsky K. Thrombopoetin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis. Blood. 1999;94(4):1273–82.
- Pagès G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouysségur J Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA 1993;90(18): 8319–23. doi: 10.1073/pnas.90.18.8319
- Qiu MS, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron 1992;9(4):705–17. doi: 10.1016/0896-6273(92)90033-A
- Eriksson M, Arminen L, Karjalainen-Lindsberg ML, Leppä S. AP-1 regulates alpha2beta1 integrin expression by ERK-dependent signals during megakaryocytic differentiation of K562 cells. Exp Cell Res 2005;304(1):175–86. doi: 10.1016/j.yexcr.2004.10.017
- Meshkini A, Yazdanparast R. Involvement of ERK/MAPK pathway in megakaryocytic differentiation of K562 cells induced by 3-hydrogenkwadaphnin. Toxicol In Vitro. 2008;22(6):1503–10. doi: 10.1016/j.tiv.2008.05.005
