Abstract
Objectives: Primary gastrointestinal diffuse large B-cell lymphoma (PGI-DLBCL) is a rare hematological malignancy with limited results on carcinogenesis and clinical characteristics. The aims of the current study were to examine mitotic arrest deficiency protein 2 (Mad2) expressions in PGI-DLBCL, and assess its association with Ki-67 expression, Helicobacter pylori (H. pylori) infection, BCL-6 gene rearrangement, and clinicopathological variables.
Methods: Cancer tissues from 38 PGI-DLBCL patients were examined for Mad2, Ki-67, and H. pylori expression by immunohistochemistry, using normal gastrointestinal tissues and nodal DLBCL as controls. BCL-6 gene translocation was analyzed by fluorescence in situ hybridization (FISH), and Mad2 expression status was evaluated along with clinicopathological characteristics.
Results: Mad2 expression was increased in PGI-DLBCL patients when compared with controls. The expression of Mad2 was 51.55 ± 22.88% in PGI-DLBCL, which was higher than reactive lymph node (28.77 ± 10.89%) and lymphoid nodule in normal gastrointestinal tissue (26.41 ± 11.30%) (P = 0.002), while it was comparable to nodal DLBCL (57.23 ± 20.79%) (P = 0.358). Mad2 overexpression had a positive correlation with Ki-67 proliferation index (r = 0.55, P = 0.01) in PGI-DLBCL, and patients with BCL-6 gene rearrangement had lower Mad2 expression (P = 0.032) than patients with intact BCL-6, while no relation was found between Mad2 expression and H. pylori infection. PGI-DLBCL patients with higher Mad2 expression had lower estimated disease-free survival (DFS) (17.10% vs. 53.00%) (P = 0.049). However, no correlation was found between Mad2 expression levels and overall survival (OS) (P = 0.443).
Conclusions: Aberrant Mad2 expression was associated with cell proliferation and genetic instability, which may contribute to the carcinogenesis of PGI-DLBCL. Mad2 overexpression indicated a poor DFS and may be a potential biomarker for estimating prognosis for PGI-DLBCL patients.
Introduction
Primary gastrointestinal lymphoma (PGIL) is a rare carcinoma accounting for approximately 1–4% of all gastrointestinal malignancies and less than 10% of all lymphomas.Citation1,Citation2 Histologically, PGIL may be mucosa-associated lymphoid tissue (MALT) lymphoma, mantle cell lymphoma, follicular lymphoma, or T-cell lymphoma, but the majority of PGILs are of diffuse large B-cell lymphoma (PGI-DLBCL) type.Citation3 The most common site of PGIL is the stomach, followed by the small intestine. The ileocoecal region, colon, esophagus, and rectum can also be involved but are relatively uncommon.Citation4 Previous studies have revealed that Helicobacter pylori (H. pylori) infection is involved in the pathogenesis of primary gastric MALT lymphoma.Citation5 Recent studies have found that the incidence of lymphoproliferative disorders, including PGIL, was increased in celiac disease patients, indicating that dysfunctional immune surveillance may be a risk factor for development of PGIL.Citation6 However, the pathogenesis remains unknown for the majority of PGIL patients.
Chromosome instability (CIN), characterized by aneuploidy, loss of heterozygosity, translocations and other chromosomal aberrations, is a hallmark of cancer cells.Citation7 The spindle assembly checkpoint (SAC) plays critical roles in maintaining genomic integrity, and SAC dysfunction has been suggested to be one of the causes of CIN, which in turn may contribute to the development of tumors. Mitotic arrest deficiency protein 2 (Mad2) is a key component of the SAC pathway and is thought to be involved in maintaining genomic stability. Aberrant Mad2 expression has been observed in a variety of human cancers and was thought to be associated with tumor initiation, progression, and prognosis.Citation8–Citation11 However, no studies on the possible pathogenic or prognostic importance of this protein in PGI-DLBCL have been reported before. In the present study, Mad2 protein expression, together with H. pylori infection, Ki-67 proliferation index (PI), and BCL-6 gene translocation were evaluated, which may be helpful to elucidate potential roles of Mad2 in the pathogenesis PGI-DLBCL, and its association with clinicopathological parameters.
Materials and methods
Patients and tissue specimens
Tumor tissues from 38 PGI-DLBCL patients were obtained by endoscopic biopsy or surgery. Fifteen reactive lymph nodes, 15 peritumoral morphologically normal gastrointestinal specimens, and 30 additional cases of nodal lymphomas (diffuse large B-cell lymphoma) were used as controls. None of the patients received chemotherapy prior to biopsy. The cohort of PGI-DLBCL patients consisted of 23 male patients and 15 females, with the average age being 56.2 years (range 28–88 years). Sites of disease include stomach (16 cases), ileocoecal region (10 cases), small intestine (6 cases), colon (5 cases), and esophagus (1 case). Patients were treated with surgery and/or CHOP based chemotherapy. The tissue specimens were fixed in 10% buffered formalin then embedded in paraffin wax. Sections were cut at 4 µm, stained with hematoxylin and eosin, and then evaluated by experienced pathologists. PGI-DLBCL diagnosis was evaluated according to Dawson's criteria.Citation12 The disease stage for PGI-DLBCL patient was determined according to the revised Ann Arbor staging system,Citation13 and the International Working Group (IWG) response criteria was used to assess the treatment results.Citation14
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue specimens were obtained for immunohistochemical test. Samples were deparaffinised in xylene, dehydrated with graded ethanol, and washed in distilled water. The sections were pretreated with microwave irradiation for antigen retrieval. To block the endogenous peroxidase activity, slides were incubated with 3% H2O2. After the blocking procedure with goat serum at room temperature for 15 minutes, sections were incubated with primary antibody against Mad2 (purified mouse anti-Mad2, BD), Ki-67, or H. pylori at a dilution of 1:100, in a humidifying chamber at 4°C overnight. Secondary antibody was added, and after incubation, the results were visualized using 3,30-diaminobenzidine. Freshly fixed normal tonsil was used as positive control and Tris-buffered saline was substituted for the primary antibody as negative control. Quantitative immunohistochemical analysis was performed by counting 1000 randomly selected malignant cells in each tissue sample showing homogenous staining intensity.
Fluorescence in situ hybridization (FISH)
FISH examination of the BCL-6 gene translocation was evaluated using the GLP BCL6 Dual Color, Break Apart Rearrangement Probe kit according to the manufacturer's instructions. The FISH results were examined on an Olympus BX51 fluorescence microscope.
Statistical analysis
The cut-off value for high and low expressions of Mad2 was determined by receiver operator characteristics (ROCs) curve. Correlation analysis was evaluated using Pearson correlation coefficients. Disease-free survival (DFS) and overall survival (OS) were estimated using the Kaplan–Meier method, and the differences in survivals were compared according to the log-rank test. SPSS 17.0 was used for data analysis and a P-value of <0.05 was considered to be statistically significant.
Results
Increased Mad2 expression was associated with cell PI in PGI-DLBCL
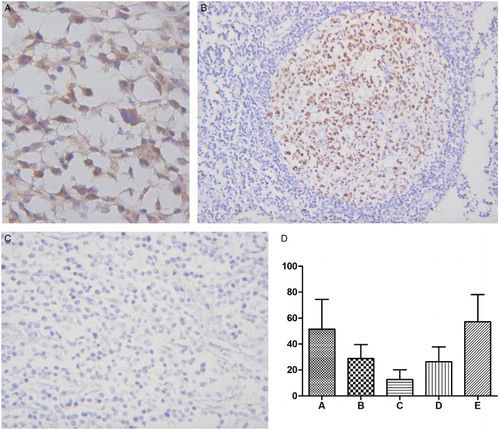
Positive signals for Mad2 showed brown-yellow nuclear or cytoplasmic staining (Fig. A). Mad2 protein expression level was 51.55 ± 22.88% (mean ± standard deviation) in PGI-DLBCL cells, which was higher than reactive lymph nodes (28.77 ± 10.89%) (P = 0.002). Nodal lymphoma of DLBCL had Mad2 expression of 57.23 ± 20.79%, which was comparable to PGI-DLBCL (P = 0.358). Lymphoid nodules in normal gastrointestinal mucosa showed Mad2 expression of 26.41 ± 11.30%, which was comparable to reactive lymph nodes, but lower than PGI-DLBCL (P = 0.002). 11.31 ± 9.00% of lamina propria cells in normal gastrointestinal tissues showed positive Mad2 staining, which was lower than PGI-DLBCL (P = 0.001). Positive and negative controls of Mad2 expression were shown in Fig. B and C, and a statistical analysis of Mad2 expression in different tissues was summarized in Fig. D.
Figure 1 Mad2 expression in PGI-DLBCL. (A) Immunohistochemical staining of Mad2. PGI-DLBCL (stomach) (400×). Mad2 was stained in the cytoplasm and nucleus of tumor cells. (B) Positive control for Mad2 expression. Normal tonsil (200×). Mad2 was mainly expressed in the germinal center. (C) Negative control for Mad2 staining. PGI-DLBCL (stomach) (400×). (D) Statistic analysis of Mad2 expression in PGI-DLBCL. A PGI-DLBCL. B Reactive lymph node (germinal center). C Reactive lymph node (non-germinal center). D Normal gastrointestinal tissue (lymphoid nodule). E Nodal lymphoma of DLBCL. (※) B–D P < 0.01, (#) E P > 0.05.
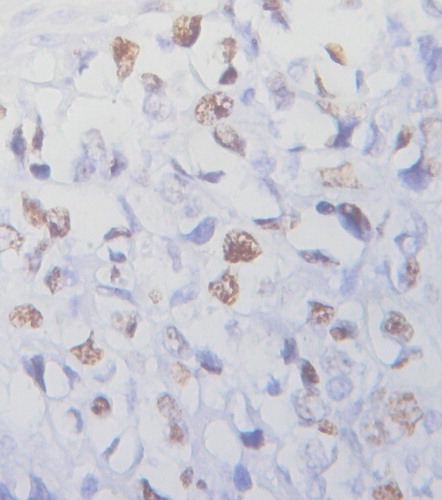
Ki-67 PI was 49.20 ± 19.27% in PGI-DLBCL cells, and 86.84% (33/38) of PGI-DLBCL patients' PI was above 30.00% (Fig. ). Since heterogeneous staining pattern of Mad2 was observed in PGI-DLBCL, a cut-off value of 46.25% of Mad2 expression was defined using the ROCs curve. According to the cut-off value, PGI-DLBCL patients were defined as high (≥46.25%) and low (<46.25%) Mad2 expression. 63.16% (24/38) of PGI-DLBCL patients were in the high Mad2 expression group and 36.84% (14/38) were in the low Mad2 expression group. PGI-DLBCL patients with high Mad2 expression had a higher Ki-67 PI than low Mad2 expression patients (58.77 ± 15.84% vs. 32.79 ± 12.42%, respectively) (P = 0.001). Correlation analysis revealed that Mad2 expression had a positive correlation with Ki-67 PI in PGI-DLBCL (r = 0.55, P = 0.01).
Figure 2 Ki-67 expression in PGI-DLBCL (ileum) (400×). Ki-67 was localized to the nucleus of PGI-DLBCL cells.
H. pylori infection was detected as well. H. pylori was expressed near the surface of tumor mass, or the residual glands around the cancer tissues. 44.74% (17/38) PGI-DLBCL patients were positive for H. pylori infection. Mad2 expression was 54.55 ± 23.06% in PGI-DLBCL patients with H. pylori infection, which was comparable to PGI-DLBCL who were H. pylori negative (49.11 ± 23.01%) (P = 0.968).
PGI-DLBCL with lower Mad2 expression had higher incidence of BCL-6 gene rearrangement
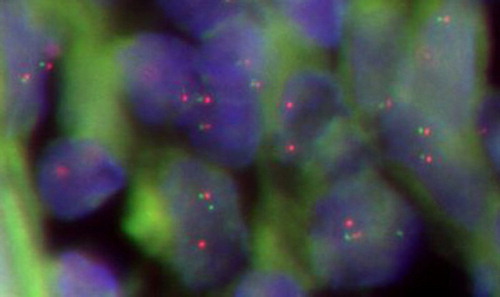
42.11% (16/38) of PGI-DLBCL patients were found positive for BCL-6 gene rearrangement using FISH analysis (Fig. ). Mad2 expression was 42.34 ± 22.17% in PGI-DLBCL patients with BCL-6 gene rearrangement, which was lower than patients who were negative for BCL-6 translocation (58.25 ± 21.44%) (P = 0.032). These results indicate that aberrant Mad2 expression may contribute to the BCL-6 gene rearrangement in PGI-DLBCL cells.
Figure 3 BCL-6 gene rearrangement in PGI-DLBCL cells (ileocecal) (400×). In normal cell hybridized with the BCL-6 probe, the expected signal pattern is two red and green fusion signals (2F). Nucleus with a translocation breakpoint involving the BCL-6 gene showing a one red, one green, and one fusion (1R1G1F) pattern.
PGI-DLBCL with higher Mad2 expression had lower disease-free survival
Mad2 expression status was compared with clinical parameters in PGI-DLBCL patients. Mad2 expression had no relation to gender, age, performance status (PS), original site or size of tumor, gross morphology, disease stage, B symptom, perforation complication, international prognosis index (IPI) score, complete remission (CR) rate, etc. Clinical characteristics of 38 PGI-DLBCL patients with high and low Mad2 expression were summarized in Table . The estimated 3 years DFS for PGI-DLBCL patients with high Mad2 expression was 17.10%, which was lower than patients with low Mad2 expression (53.00%) (P = 0.049) (Fig. ). The estimated 3 years OS for PGI-DLBCL with high Mad2 expression was 61.10%, which was lower than patients with low Mad2 expression (78.60%), but the difference was not significant (P = 0.443). No correlation was found between Mad2 expression and OS.
Figure 4 Disease-free survival of patients with PGI-DLBCL. Upper line: low Mad2 expression; lower line: high Mad2 expression.
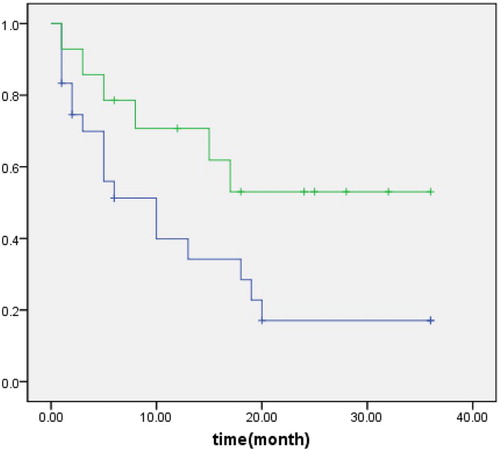
Table 1 Clinical characteristics of 38 patients with PGI-DLBCL
Discussion
Although PGIL is relatively a rare hematological malignancy, it is the most common site of extranodal lymphomas, with increasing incidence worldwide.Citation15 The clinical features of PGIL are not specific and may be indistinguishable from other gastrointestinal cancers. Combinations of surgical treatment and/or chemotherapy are widely used in clinical settings, although surgical resection has no specific effect on long-term survival.Citation16 Nevertheless, previous observations indicated that the treatment outcomes of PGIL were generally better than that of nodal lymphoma.Citation17
CIN is a classical feature of tumor cells and is usually associated with poor prognosis. SAC dysfunction has been suggested as a contributor to CIN and carcinogenesis. The components of the SAC pathway consist of evolutionarily conserved proteins including Mad2. Mad2 is a key component of SAC, and it functions by binding to its mitotic-specific activator, Cdc20, and then delays the onset of anaphase.Citation18 Previous study indicated that Mad2 overexpression was sufficient to cause CIN in vitro and in vivo.Citation19 In addition, aberrant expression of Mad2 is thought to be associated with CIN and contribute to the development of cancer.Citation20,Citation21 Aberrant regulation of Mad2 expression may facilitate tumor progression and metastasis as well.Citation22 However, the importance of these spindle proteins remains largely unknown in the carcinogenesis of PGIL.
As the most common type of PGIL, PGI-DLBCL patients are subjected to Mad2 expression examination. Mad2 had a higher level of expression in PGI-DLBCL cells when compared with normal gastrointestinal tissue and reactive lymph node. Furthermore, overexpression of Mad2 was positively correlated with Ki-67 proliferation index. On the other hand, PGI-DLBCL patients with lower Mad2 expression have a higher incidence of BCL-6 gene rearrangement, indicating that Mad2 may be needed for maintaining genome stability. PGI-DLBCL patients with higher Mad2 expression exhibited poorer DFS, suggested that increased expression of Mad2 might promote tumor progression in PGI-DLBCL, and Mad2 expression may be a potential prognostic factor for PGI-DLBCL patients. Although patients with high Mad2 expression showed worse OS than those with low Mad2 expression, the difference was not significant, which may be due to small sample size.
In summary, aberrant Mad2 expression is correlated with high cell proliferation and BCL-6 gene translocation in PGI-DLBCL, suggesting that Mad2 protein may be one of the contributing factors involved in the carcinogenesis of PGI-DLBCL. Mad2 overexpression may be a potential biomarker for estimating a poor DFS in patients with PGI-DLBCL.
Disclaimer statements
Contributors All authors have contributed to this work.
Funding This work was supported by grants from the Natural Science Foundation of Hubei Province (2013CFB266) (F.C).
Conflicts of interest There are no conflicts of interest regarding this paper.
Ethics approval The institutional review board of Zhongnan Hospital of Wuhan University has approved this study.
References
- Cooper DL, Doria R, Salloum E. Primary gastrointestinal lymphomas. Gastroenterologist. 1996;4(1):54–64.
- Ghimire P, Wu GY, Zhu L. Primary gastroin-testinal lymphoma. World J Gastroenterol. 2011;17(6):697–707. doi: 10.3748/wjg.v17.i6.697
- O'Malley DP, Goldstein NS, Banks PM. The recognition and classification of lymphoproliferative disorders of the gut. Hum Pathol. 2014;45(5):899–916. doi: 10.1016/j.humpath.2013.12.001
- Amer MH, El-Akkad S. Gastrointestinal lymphoma in adults: clinical features and management of 300 cases. Gastroenterology. 1994;106(4):846–58.
- Stolte M. Helicobacter pylori gastritis and gastric MALT-lymphoma. Lancet. 1992;339(8795):745–6. doi: 10.1016/0140-6736(92)90645-J
- Leslie LA, Lebwohl B, Neugut AI, Gregory MJ, Bhagat G, Green PH. Incidence of lymphoproliferative disorders in patients with celiac disease. Am J Hematol. 2012;87(8):754–759. doi: 10.1002/ajh.23237
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013
- Hisaoka M, Matsuyama A, Hashimoto H. Aberrant MAD2 expression in soft-tissue sarcoma. Pathol Int. 2008;58(6):329–333. doi: 10.1111/j.1440-1827.2008.02232.x
- Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kondo S, et al. Overexpression of MAD2 predicts clinical outcome in primary lung cancer patients. Lung Cancer. 2011;74(1):124–131. doi: 10.1016/j.lungcan.2011.01.025
- Li L, Xu DB, Zhao XL, Hao TY. Combination analysis of Bub1 and Mad2 expression in endometrial cancer: act as a prognostic factor in endometrial cancer. Arch Gynecol Obstet. 2013;288(1):155–165. doi: 10.1007/s00404-012-2706-7
- Choi JW, Kim Y, Lee JH, Kim YS. High expression of spindle assembly checkpoint proteins CDC20 and MAD2 is associated with poor prognosis in urothelial bladder cancer. Virchows Arch. 2013;463(5):681–687. doi: 10.1007/s00428-013-1473-6
- Dawson IMP, Cornes JS, Morson BC. Primary malignant lymphoid tumors of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49(1):80–89. doi: 10.1002/bjs.18004921319
- Musshoff K. Clinical staging classification of non-Hodgkin's lymphomas. Strahlentherapie. 1997;153(4):218–221.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403
- Howell JM, Auer-Grzesiak I, Zhang J, Andrews CN, Stewart D, Urbanski SJ. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol. 2012;26(7):452–456.
- Cheung MC, Housri N, Ogilvie MP, Sola JE, Koniaris LG. Surgery does not adversely affect survival in primary gastrointestinal lymphoma. J Surg Oncol. 2009;100(1):59–64. doi: 10.1002/jso.21298
- Fischbach W. Gastrointestinal lymphomas: the Wurzburg study experience. Recent Results Cancer Res. 2000;156:134–140. doi: 10.1007/978-3-642-57054-4_17
- Han JS, Holland AJ, Fachinetti D, Kulukian A, Cetin B, Cleveland DW. Catalytic assembly of the mitotic checkpoint inhibitor BubR1-Cdc20 by a Mad2-induced functional switch in Cdc20. Mol Cell. 2013;51(1):92–104. doi: 10.1016/j.molcel.2013.05.019
- Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, et al. Mad2 Overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11(1):9–23. doi: 10.1016/j.ccr.2006.10.019
- Gemma A, Hosoya Y, Seike M, Uematsu K, Kurimoto F, Hibino S, et al. Genomic structure of the human MAD2 gene and mutation analysis in human lung and breast cancers. Lung Cancer. 2001;32(3):289–295. doi: 10.1016/S0169-5002(00)00223-3
- Sotillo R, Schvartzman JM, Socci ND, Benezra R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature. 2010;464(7287):436–440. doi: 10.1038/nature08803
- Yu L, Liu S, Guo W, Zhang B, Liang Y, Feng Q. Upregulation of Mad2 facilitates in vivo and in vitro osteosarcoma progression. Oncol Rep. 2012;28(6):2170–2176.
