Abstract
Objectives: Although additional sex comb-like 1 (ASXL1) gene mutations have long been reported in myelodysplastic syndromes (MDSs) and chronic myelomonocytic leukemia (CMML), the prognostic significance has been controversial. Therefore, a meta-analysis to study the impact of ASXL1 mutations on patients with MDS and CMML is useful.
Methods: The identified articles were retrieved from some common databases. We extracted hazard ratios (HRs) for overall survival (OS) and leukemic-free survival (LFS) and P-value of some clinical parameters, which compared AXSL1 mutations to those without from the available studies. Each individual HR and P-value was used to calculate the pooled HR and P-value.
Results: Six studies covering 1689 patients were selected for this meta-analysis. The pooled HRs for OS and LFS were 1.45 (95% confidential interval (CI), 1.24–1.70) and 2.20 (95% CI, 1.53–3.17), respectively. When considering CMML patients alone the HR for OS was 1.50 (95% CI, 1.18–1.90). Additionally, ASXL1 mutations were more frequently found in male (P = 0.008), older (P = 0.019), and patients with lower platelets (P = 0.009) or hemoglobin level (P = 0.0015) and associated with other mutations such as EZH2, IDH1/2, RUNX1, and TET2.
Discussion: Although our analysis has its limitation, it showed that ASXL1 mutations had significant inferior impact on OS and LFS for French–American–British-defined MDS patients. However, the influence of different types of ASXL1 mutations on patients with MDS still needs illustrating.
Conclusion: ASXL1 mutations were associated with poor prognosis in MDS, which may contribute to risk stratification and prognostic assessment in the disease.
Introduction
The myelodysplastic syndromes (MDSs) are a heterogeneous clonal disease originating in hematopoietic stem cell and characterized by myeloid dysplasia, impaired differentiation, blood cytopenia, and a predisposition to acute myeloid leukemia (AML).Citation1–Citation3 Chronic myelomonocytic leukemia (CMML) belongs to MDS according to French–American–British (FAB) classification. Currently, since that CMML is a disease with features of both myelodysplasia and myeloproliferative, a new classification system proposed by the World Health Organization (WHO) in 2002 places it to MDS/myeloproliferative category.Citation4 However, to date, no cytogenetic or molecular abnormality is unique to a particular subtype of CMML and clinical studies still divide patients according to the FAB classification.Citation4 So, in this report, we focused on the influence of ASXL1 mutations on clinical prognosis of MDS and CMML patients.
Recently, with the development of next-generation sequencing, it is possible to identify new somatic mutations that may play important roles in both pathogenesis and clinical prognosis in MDS and CMML patients. ASXL1 gene mutations are one of the representative mutations. ASXL1 gene is located in chromosome band 20q11Citation5 and encodes a highly conserved protein that belongs to the enhancer of trithorax and polycomb (ETP) genes.Citation6–Citation10 The function of this protein remains to be defined in humans. Some researches indicated that it was involved in regulation of histone methylation and could act as a ligand-dependent coactivator for receptor through binding with steroid receptor coactivator-1.Citation7 It is one of the most frequently mutated gene found to have prognostic significance independent of known clinical risk factors in several prior studies.Citation11–Citation26 Despite that ASXL1 gene mutations were first reported in MDS,Citation2 the function and prognosis of them in MDS and CMML were still in dispute. Many studies focused on the clinical impact of ASXL1 gene mutations in MDS and CMML have reported variable results, most of which indicated that the ASXL1 mutations were associated with adverse prognosis, but not all found that they were independent prognostic factors,Citation27–Citation34 some of them even considered them to have no impact on outcome.Citation34,Citation35 Therefore, in order to gain a full insight into the prognostic value of ASXL1 mutations in patients with MDS and CMML, we conducted a meta-analysis.
Materials and methods
Study Selection
A systematic literature search of Medline, EMBASE, PubMed, The Cochrane Library, Web of Science, CMJD (Chinese Medical Journal Database), and the Chinese Nation Knowledge Infrastructure (CNKI) was performed by two independent reviewers (Y.L. and Z.Y.). Relevant papers published between 2005 and 2015 were obtained by using the search terms ‘MDS OR “myelodysplastic syndrome” OR “myelodysplasia” OR “preleukemia” OR CMML OR “chronic myelomonocytic leukemia”’ and ‘ASXL1 OR “additional sex comb-like 1”’. The search was restricted to human studies, free articles with no language limitation. Independent search terms were used to search Chinese databases. We also reviewed the references for missing information.
The researched papers included prospective or retrospective or clinical trials meeting all the following criteria were eligible in this meta-analysis: (1) published between 2005 and 2015 as original articles; (2) assessed the association between ASXL1 mutations and outcomes in MDS and CMML; (3) offered detailed survival information of patients with ASXL1 mutations, such as overall survival (OS), leukemia-free survival (LFS), and the corresponding hazard ratios (HRs) as well as 95% confidential intervals (CIs) and P-value. End points of OS were measured from the date of first sample collection to the time of death from any causes or to the time at last follow-up (censored). LFS was calculated from date of first sample collection to the time of AML diagnosis. If HRs and 95% CIs were not available, we calculated them according to the reported Kaplan–Meier or P-value or other statistical parameters given in the text using the method proposed by Parmar et al.Citation36 and Hotta et al.Citation37 If the author reported both univariate analysis and multivariate analysis to get the HR, the result of multivariate analysis including other variables (the more the better as presumed) should be preferably taken because it could be more accurate.
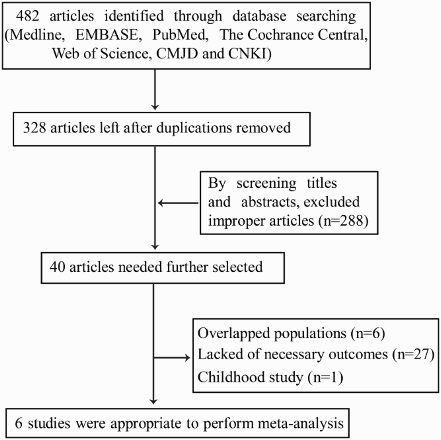
Four hundred and eighty-two papers were received by the search strategy. By screening the titles and reviewing abstracts, animal studies, letters to the editor, reviews, case reports, duplicate publications, and other articles that did not meet the selection criteria were excluded. Then, there were still 40 articles needed further screening. The selection process is shown in Fig. . Finally, six studies were included in the meta-analysis.Citation27–Citation31,Citation35
Date extraction
To mostly reduce bias, two reviewers (Y.L. and Z.Y) independently extracted the following information from the included studies (Table ): First Author's name, Journal, year of publication, region, total patients, number of ASXL1 mutations, age and gender distribution of patients, criteria for classification of MDS and CMML, karyotypes, and International Prognostic Scoring System (IPSS) classificationCitation28 (Table ). HR and 95% CI for OS and LFS were also extracted from the included studies. Efforts were made to contact corresponding authors for missing data.
Table 1 Summary of the data extracted from the six studies included
Table 2 Determination of IPSS risk groups
Quality assessment
The quality of evidence and the strength of recommendations were assessed using the Newcastle–Ottawa quality assessment (NOS) for cohort studies for each individual study. The NOS has nine items categorized into three major categories: selection (four items), comparability (two items), and outcome (three items). There were stars marked in 10 of the criteria in this assessment. If the studies met one standard, they got one star (point), otherwise none. The total score was 10.Citation38 We considered the final score of each study 6 or more as high quality. Any discrepancies were resolved among authors.
Statistical analysis
All statistical analyses were performed using Stata ver.12 software (College Station, TX, USA). HRs and 95% CIs of OS and LFS were used to assess the prognostic effect of ASXL1 mutations compared with wild type in MDS and CMML patients. We also conducted a subgroup analysis in CMML patients. The statistical heterogeneity of the effect was assessed by the I2 and Q statistics (I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity).Citation39 Data were to be calculated using fix-effects models when the heterogeneity was moderate or below. Otherwise, the random-effects model was used. Begg and Mazumdar'sCitation40 and the Egger et al.'sCitation22 tests were used to assess possible publication bias. We also calculated the overall P-value in age, gender, platelets count, and hemoglobin level between ASXL1 mutated and ASXL1 wild type patients. A two-tailed P-value of less than 0.05 was defined as statistically significant. All the statistical analyses were done by Y.L. and Y.Z.
Results
Characteristics of the selected studies
As shown in Fig. , six studies covering a total of 1689 patients were included in the present analysis. Characteristic of them were listed in Table . Three studies of them were from the United States,Citation28,Citation31,Citation35 one from France,Citation30 one from Germany,Citation29 and another one from Taiwan. Three of the studies focused on CMML patients,Citation30,Citation31,Citation35 one focused on MDS according to WHO classification, and the rest two studies enrolled both MDS and CMML patients. Among the 1689 patients, 446 carried ASXL1 mutations and 947 had detailed information of cytogenetic characteristics which were grouped into three categories according to the IPSS-based karyotypes: good, intermediate, and poor.
The frequency of ASXL1 mutations in MDS (14.4–22.7%) and CMML (14.4–47.2%) was similar to others reported before. Several studiesCitation27,Citation28,Citation30,Citation31 reported that ASXL1 mutations seemed to be independent adverse prognostic factors for OS and LFS of CMML and MDS patients; however, Thol et al.Citation29 study suggested ASXL1 mutations were independent unfavorable prognostic factor for OS when considering only frameshift mutations, when considering both frameshift and missense mutations, ASXL1 had no independent impact on OS. Also, Patnaik et al.Citation35 found ASXL1 mutations had no impact on survival of CMML patients. Beyond that, ASXL1 mutations were found to coincide with other mutations such as IDH1/2, NPM1, RUNX1, and TET2 (Table ). The results of quality assessment displayed the evidence and the strength of individual study was high. The NOS scores are listed in Table .
Table 3 Combined mutations of ASXL1 with IDH1/2, NPM1, RUNX1, and TET2 in MDSs
Table 4 Quality assessment of individual study
Outcomes of the meta-analysis
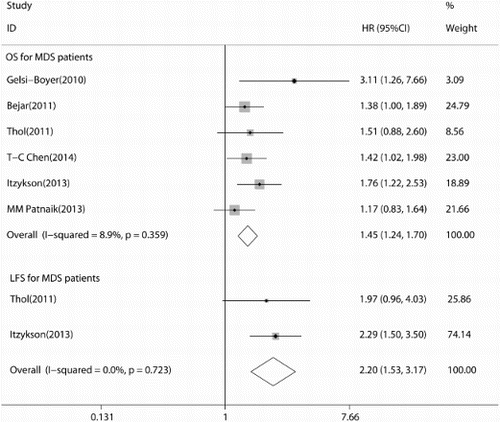
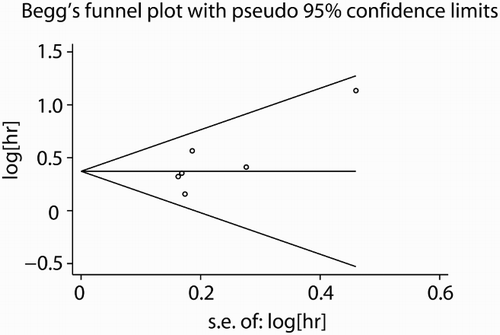
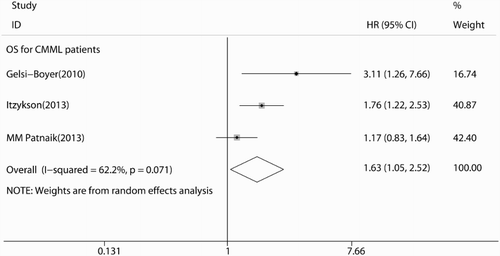
As shown in Fig. , the summary HR in OS of overall patients was 1.45 (95% CI: 1.24–1.70, P < 0.001), which indicated that ASXL1 mutations were poor factors for OS of FAB-defined MDS patients. Because of the limited information in LFS, we only performed an analysis of LFS in Thol (2011)Citation29 and Itzykson (2013)Citation31 studies. The overall HRs were 2.20 (95% CI: 1.53–3.17, P < 0.001), also suggested ASXL1 mutations had poor impact on LFS of FAB-defined MDS patients (Fig. ). Additionally, we conducted sensitivity analysis by omitting one individual study at a time to measure its effect on pooled HR. Results showed that there was no heterogeneity among the studies. Meanwhile, the Begg's and Egger's tests were used to examine publication bias (Begg's P = 0.133; Egger's P = 0.111; Fig. ). There was no evidence for significant publication bias in this meta-analysis. We also calculated the overall P-value in age, gender, platelets count, and hemoglobin level between ASXL1 mutated and ASXL1 wild type patients. Consistent with Chen (2014),Citation27 Gelsi-Boyer (2010),Citation30 and Patnaik (2013)Citation35 studies, ASXL1 mutations were more frequently found in male patients (P = 0.008), older patients (P = 0.019), and patients with lower platelets (P = 0.009) or hemoglobin level (P = 0.0015). As shown in Patnaik et al.Citation35 (P = 0.27) and Thol et al.Citation29 (P = 0.48) studies, there was no significant difference for bone marrow blast proportion between ASXL1 mutated and ASXL1 wild type patients. Furthermore, subgroup analysis of ASXL1 mutations on CMML patentsCitation28,Citation31,Citation35 was performed. The results showed that there existed moderate heterogeneity (I2 = 62.2%) in the analysis and the pooled HR was 1.50 (95% CI: 1.18–1.90), which indicated that ASXL1 mutations also associated with poor OS outcome in CMML patients alone (Fig. ).
Discussion
Recently, with the widespread use of next-generation sequencing, makes it possible to identify new somatic mutations that have potential impact on myeloid malignancies. ASXL1 mutations are the representative. Although several study centers have assessed the prognostic implication of ASXL1 mutations in MDS and CMML patients,Citation27–Citation34 the results are inconsistent to some extent. Meanwhile, most of the studies are single-center non-randomized trial with small sample sizes and lack high-quality evidence. That is to say, the exact relation between ASXL1 mutations and prognosis of MDS and CMML patients still needed illuminating. Since meta-analysis is a useful statistical method for integrating results from independent studies and can reduce bias that may be caused by missing information in individual studies, we perform a meta-analysis to exactly delineate the prognostic role of ASXL1 mutations in MDS and CMML patients. In this meta-analysis, six trials covering a number of 1689 from different study centers were included. The findings were as follows: ASXL1 mutations were found to be independent adverse prognostic factors both in OS and LFS on MDS patients based on FAB classification. In addition, we found that ASXL1 mutations associated with shorter OS HR (1.50, 95% CI: 1.18–1.90) in CMML patients alone. The results were consistent with most studies before.Citation27–Citation29,Citation31 Besides, as with AML, ASXL1 mutations were more frequently found in maleCitation42–Citation46 patients, or older age patients,Citation14,Citation42–Citation46 or patients with lower platelets and hemoglobin level, which may somehow explain why ASXL1 mutations indicated poor impact in MDS patients. Although bone marrow blast proportion was related with poor prognosis, we found no significant difference between ASXL1 mutated and ASXL1 wild type patients from included studies. Due to lack of data, we were unable to assess other clinical parameters.
As we know, ASXL1 gene maps to chromosome 20q11, a region frequently amplified in human tumors and encodes a highly conserved protein that belongs to the ETP gene and is associated with both epigenetic activation and repression of gene transcription.Citation8 However, the exact functions of ASXL1 remain to be defined in human. Some studies demonstrated that ASXL1 mutations coincided with many known gene mutations including EZH2,Citation41 IDH1/2, RUNX1, and TET2,Citation15,Citation28 most of which were confirmed as adverse prognostic factors in malignant myeloid diseases, this may also explain why ASXL1 mutations associated with poor prognosis in MDS patient to some extent. Of course, the exact mechanisms need to be further elucidated. We also know, ASXL1 mutations included frameshift, nonsense, and missense mutations, and frameshift mutations were most frequently happened, especially in c.1934dup;p.G646WfsX12. Frameshift and nonsense mutations are reported to result in C-terminal truncation of the protein upstream of the Plant homeodomain finger, but the functional relevance of some reported missense mutations is not clear.Citation11 According to Thol et al.Citation29 study, only frameshift mutations associated with poor prognosis in MDS patients; however, when considering both missense mutations and frameshift mutations, ASXL1 mutations had no independent impact on OS. All studies in this meta-analysis included both frameshift mutations and other mutations and the results were significant. Due to the lack of data, subgroup analysis focus on the mutation types of ASXL1 gene was restrained. It was not sure whether ASXL1 missense mutations had different impact on MDS patients with frameshift mutations. Additional studies needed to be conducted to explain the exact influence of ASXL1 missense on prognosis of patients with MDS.
Although our analysis had its limitation, firstly, all the enrolled studies were observational rather than prospective randomized controlled studies; secondly, the analysis covered a small number of MDS and CMML patients; beyond that, we could not avoid potential heterogeneity and publication bias in the meta-analysis. However, it showed that ASXL1 mutations had significant inferior impact on OS and LFS for FAB-defined MDS patients. Furthermore, we found that ASXL1 mutations coincided with IDH1/2, NPM1, RUNX1, and TET2. In conclusion, the detection of ASXL1 mutations was useful to assess prognosis and guide treatment in MDS patients. Maybe, a new prognostic score system including ASXL1 mutations will be created to replace the traditional IPSS risk groups, however, before that, prospective randomized controlled studies covering a large numbers and investigating the biologic role and different types of ASXL1 mutations in MDS and CMML patients are needed.
Disclaimer statements
Contributors Y.L. designed and written this article. Y.Z. helped to modify and improve tables and figures. Z.-C.W. assisted in dealing with statistical problems. S.-Y.W. was the advisor.
Funding None.
Conflicts of interest All authors have no conflicts of interest to report.
Ethics approval Our study did not need ethical approval.
Acknowledgments
We thank all patients and clinical investigators who were involved in the studies selected for this meta-analysis. This work was supported by grants from the National Natural Science Foundation of China (81270609, 30770909, and 81470008), the Major Science & Technology Project of Fujian Province (2003F003, 2012Y4012), the Major Science & Technology Project of Fujian Medical University (09ZD008), and the National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, P.R.C.
References
- Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–51. doi: 10.1182/blood-2007-08-078139
- Nolte F, Hofmann WK. Myelodysplastic syndromes: molecular pathogenesis and genomic changes. Ann Hematol. 2008;87:777–95. doi: 10.1007/s00277-008-0502-z
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. doi: 10.1056/NEJMra0902908
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199
- Fisher CL, Berger J, Randazzo F, Brock HWA. Human homolog of additional sex combs, additional sex combs-like 1, maps to chromosome 20q11. Gene. 2003;306:115–26. doi: 10.1016/S0378-1119(03)00430-X
- Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, et al. Histone H2A deubiquitinase activity of the polycomb repressive complex PR-DUB. Nature. 2010;465:243–7. doi: 10.1038/nature08966
- Cho YS, Kim EJ, Park UH, Sin HS, Um SJ. Additional sex comb-like 1 (ASXL1), in cooperation with SRC-1, acts as a ligand-dependent coactivator for retinoic acid receptor. J Biol Chem. 2006;281:17588–98. doi: 10.1074/jbc.M512616200
- Fisher CL, Randazzo F, Humphries RK, Brock HW. Characterization of Asxl1, a murine homolog of additional sex combs, and analysis of the Asx-like gene family. Gene. 2006;369:109–18. doi: 10.1016/j.gene.2005.10.033
- Lee SW, Cho YS, Na JM, Park UH, Kang M, Kim EJ, et al. ASXL1 represses retinoic acid receptor-mediated transcription through associating with HP1 and LSD1. J Biol Chem. 2010;285:18–29. doi: 10.1074/jbc.M109.065862
- Fisher CL, Pineault N, Brookes C, Helgason CD, Ohta H, Bodner C, et al. Loss-of-function additional sex combs like 1 mutations disrupt hematopoiesis but do not cause severe myelodysplasia or leukemia. Blood. 2010;115:38–46. doi: 10.1182/blood-2009-07-230698
- Gelsi-Boyer V, Trouplin V, Adelaide J, Bonansea J, Cervera N, Carbuccia N, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145:788–800. doi: 10.1111/j.1365-2141.2009.07697.x
- Boultwood J, Perry J, Pellagatti A, Fernandez-Mercado M, Fernandez-Santamaria C, Calasanz MJ, et al. Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia. 2010;24:1062–5. doi: 10.1038/leu.2010.20
- Abdel-Wahab O, Pardanani A, Patel J, Wadleigh M, Lasho T, Heguy A, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25:1200–2. doi: 10.1038/leu.2011.58
- Pratcorona M, Abbas S, Sanders M, Koenders J, Karelaars F, Erpelinck-Verschueren C, et al. Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haematologica. 2012;97:388–92. doi: 10.3324/haematol.2011.051532
- Rocquain J, Carbuccia N, Trouplin V, Raynaud S, Murati A, Nezri M, et al. Combined mutations of ASXL1, CBL, FLT3, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2 and WT1 genes in myelodysplastic syndromes and acute myeloid leukemias. BMC Cancer. 2010;10:401–7. doi: 10.1186/1471-2407-10-401
- Brecqueville M, Rey J, Bertucci F, Coppin E, Finetti P, Carbuccia N, et al. Mutation analysis of ASXL1, CBL, DNMT3A, IDH1, IDH2, JAK2, MPL, NF1, SF3B1, SUZ12 and TET2 in myeloproliferative neoplasms. Genes Chromosomes Cancer. 2012;51:743–55. doi: 10.1002/gcc.21960
- Boultwood J, Perry J, Zaman R, Fernandez-Santamaria C, Littlewood T, Kusec R, et al. High-density single nucleotide polymorphism array analysis and ASXL1 gene mutation screening in chronic myeloid leukemia during disease progression. Leukemia. 2010;24:1139–45. doi: 10.1038/leu.2010.65
- Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adélaïde J, Rey J, et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia. 2009;23:2183–6. doi: 10.1038/leu.2009.141
- Chou WC, Huang HH, Hou HA, Chen CY, Tang JL, Yao M, et al. Distinct clinical and biological features of de novo acute myeloid leukemia with additional sex comblike 1 (ASXL1) mutations. Blood. 2011;116:4086–94. doi: 10.1182/blood-2010-05-283291
- Stein BL, Williams DM, O'Keefe C, Rogers O, Ingersoll RG, Spivak JL, et al. Disruption of the ASXL1 gene is frequent in primary, post-essential thrombocytosis and postpolycythemia vera myelofibrosis, but not essential thrombocytosis or polycythemia vera: analysis of molecular genetics and clinical phenotypes. Haematologica. 2011;96:1462–9. doi: 10.3324/haematol.2011.045591
- Shen Y, Zhu YM, Fan X, Shi JY, Wang QR, Yan XJ, et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood. 2011;118:5593–603. doi: 10.1182/blood-2011-03-343988
- Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, et al. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118:3932–41. doi: 10.1182/blood-2010-10-311019
- Abdel-Wahab O, Manshouri T, Patel J, Harris K, Yao J, Hedvat C, et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010;70:447–52. doi: 10.1158/0008-5472.CAN-09-3783
- Carbuccia N, Trouplin V, Gelsi-Boyer V, Murati A, Rocquain J, Adélaïde J, et al. Mutual exclusion of ASXL1 and NPM1 mutations in a series of acute myeloid leukemias. Leukemia. 2010;24:469–73. doi: 10.1038/leu.2009.218
- Grossmann V, Kohlmann A, Eder C, Haferlach C, Kern W, Cross NC, et al. Molecular profiling of chronic myelomonocytic leukemia reveals diverse mutations in >80% of patients with TET2 and EZH2 being of high prognostic relevance. Leukemia. 2012;25:877–9. doi: 10.1038/leu.2011.10
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629
- Chen T-C, Houl H-A, Chou W-C, Tang J-L, Kuo Y-Y, Chen C-Y, et al. Dynamics of ASXL1 mutation and other associated genetic alterations during disease progression in patients with primary myelodysplastic syndrome. Blood Cancer J. 2014;4:e177. doi: 10.1038/bcj.2013.74
- Bejar R, Stevenson K, Abdel-Wahab O, Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506. doi: 10.1056/NEJMoa1013343
- Thol F, Friesen I, Damm F, Yun H, Eva M, Weissinger E, et al. Prognostic significance of ASXL1 mutations in patients with myelodysplastic syndromes. J Clin Oncol. 2011;29:2499–506. doi: 10.1200/JCO.2010.33.4938
- Gelsi-Boyer V, Trouplin V, Roquain J, Adélaïde J, Carbuccia N, Esterni B, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol. 2010;151:365–75. doi: 10.1111/j.1365-2141.2010.08381.x
- Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31:2428–36. doi: 10.1200/JCO.2012.47.3314
- Bacher U, Haferlach T, Schnittger S, Zenger M, Meggendorfer M, Jeromin S, et al. Investigation of 305 patients with myelodysplastic syndromes and 20q deletion for associated cytogenetic and molecular genetic lesions and their prognostic impact. Br J Haematol. 2014;164:822–33. doi: 10.1111/bjh.12710
- Thol F, Winschel C, Lüdeking A, Yun H, Friesen I, Damm F, et al. Rare occurrence of DNMT3A mutations in myelodysplastic syndromes. Haematologica. 2011;96:1870–3. doi: 10.3324/haematol.2011.045559
- Wang J, Ai X, Gale RP, Xu Z, Qin T, Fang L, et al. TET2, ASXL1 and EZH2 mutations in Chinese with myelodysplastic syndromes. Leuk Res. 2013;37:305–11. doi: 10.1016/j.leukres.2012.10.004
- Patnaik MM, Padron E, LaBorde RR, Lasho TL, Finke CM, Hanson CA, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27:1504–10. doi: 10.1038/leu.2013.88
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:3852–9. doi: 10.1200/JCO.2004.02.109
- Ottawa Hospital Research Institute. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446
- Abdel-Wahab O, Pardanani A, Patel J, Wadleigh M, Lasho T, Heguy A, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25:1200–2. doi: 10.1038/leu.2011.58
- Metzeler KH, Becker H, Maharry K, Radmacher MD, Kohlschmidt J, Mrózek K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytogenetically normal AML within the ELN favorable genetic category. Blood. 2011;118:6920–9. doi: 10.1182/blood-2011-08-368225
- Shivarov V, Gueorguieva R, Ivanova M, Tiu RV. ASXL1 mutations define a subgroup of AML patients with distinct gene expression profile and poor prognosis: a meta-analysis of 3311 adult AML patients. Leuk Lymphoma. 2015;56:1881–3. doi: 10.3109/10428194.2014.974596
- Chou WC, Huang HH, Hou HA, Chen CY, Tang JL, Yao M, et al. Distinct clinical and biological features of de novo acute myeloid leukemia with additional sex comb-like 1 (ASXL1) mutations. Blood. 2010;116:4086–94. doi: 10.1182/blood-2010-05-283291
- El-Sharkawi D, Ali A, Evans CM, Hills RK, Burnett AK, Linch DC, et al. ASXL1 mutations are infrequent in young patients with primary acute myeloid leukemia and their detection has a limited role in therapeutic risk stratification. Leuk Lymphoma. 2014;55:1326–31. doi: 10.3109/10428194.2013.833332
- Schnittger S, Eder C, Jeromin S, Alpermann T, Fasan A, Grossmann V, et al. ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome. Leukemia. 2013;27:82–91. doi: 10.1038/leu.2012.262