Abstract
Objective: Many patients with psoriasis have developed acute promyelocytic leukemia (APL) whereas few reports on psoriasis-associated APL were found in the published literature. This study was aimed to study the etiology, clinical characteristics, and prognosis of psoriasis-associated APL and to map a suitable treatment regime for this condition.
Methods: This study retrospectively analyzed the clinical data of 17 patients with psoriasis-associated APL diagnosed and treated in our hospital in the past decade.
Results: The 17 patients accounted for 8.3% of the total patients diagnosed with de novo APL during the same period in our hospital. Their clinical characteristics of APL were similar to those of general APL. Four patients had a definite history of taking bimolane. All patients received arsenic trioxide (ATO)-based remission induction and postremission treatment. After induction, 15 patients (88%) achieved hematologic complete remission. With a median follow-up of 27 months, the 3-year estimates of overall survival were 77.2% ± 12.4% and the 3-year estimates of event-free survival were 70.6% ± 13.5%. In addition, the ATO-based remission induction and postremission treatment significantly improved psoriasis symptoms in 83 and 85.7% of patients, respectively. Through the final follow-up, no chronic arsenicosis or secondary malignancy was observed.
Conclusions: Psoriasis patients are at high risk for APL. The increased risk is most likely associated with the genetic background and bimolane treatment. The ATO-based therapy is especially suitable for patients with psoriasis-associated APL. Our study also brings a new treatment option for psoriasis.
Introduction
Psoriasis is a common, inflammatory skin disease which is characterized by abnormal keratinocyte proliferation and differentiation as well as prominent immune cell infiltration, and its pathogenesis remains unclear. Now it is generally accepted that psoriasis is a T-cell-mediated autoimmune disorder and there is a strong genetic basis for its development. Although there are a variety of treatments for psoriasis, such as topical corticosteroid, topical vitamin D3 analog, oral cyclosporine, phototherapy, and biological treatments, there remains no way to cure the condition and there will always be some patients resistant to treatment of any kind. Therefore, new therapeutic strategies or drugs still need to be explored.
Acute promyelocytic leukemia (APL) is a specific subtype of acute myeloid leukemia (AML); in the majority of cases, there is a t(15;17) chromosomal translocation that causes formation of the promyelocytic leukemia–retinoic acid receptor-α (PML-RARα) fusion gene. The differentiation therapy using all-trans-retinoic-acid and arsenic trioxide (ATO) has yielded great success for patients with APL. All-trans-retinoic-acid and ATO can specifically target the RARα and PML moieties of fusion proteins, respectively, to cause degradation of the fusion proteins.
Numerous reportsCitation1–Citation3 have shown that many psoriasis patients, with or without a history of drug treatment, developed acute leukemia, of which approximately one-half was APL. However, to our knowledge till now, few reports focused on psoriasis-associated APL were found in the published literature.Citation4
Here, we retrospectively analyzed the clinical data of patients with psoriasis-associated APL who were diagnosed and treated in our hospital in the past decade. This study was aimed to study the etiology, clinical characteristics, and prognosis of psoriasis-associated APL and to map a suitable treatment regime for this condition, as well as to provide a new treatment option for psoriasis.
Methods
Patients
In total, 283 patients were diagnosed with de novo APL in the First Affiliated Hospital of Harbin Medical University between January 2005 and August 2014, of whom 204 had positive detection results of t(15,17) and/or PML-RARα fusion gene in their bone marrow samples. Nineteen cases of psoriasis-associated APL patients were discovered, of whom 17 patients (8.3%) had positive detection results for t(15,17) and/or the PML-RARα fusion gene in the bone marrow. Written informed consent was required, and the study protocol was reviewed and approved by the hospital's Medical Ethics Committee.
Treatment
All 17 patients were intravenously administered ATO (10 mg/day) for induction therapy until achievement of hematologic complete remission (HCR). All patients who achieved HCR received almost 3–4 years of postremission therapy. Two different postremission treatment regimes (Table ) were used based on physician preference. In one treatment regime, single-agent ATO was regularly used for 4 years (treatment regimen A), while in the other, three courses of daunorubicin or idarubicin + cytarabine (DA) were given, followed by regular use of ATO for 3 years (treatment regimen B).
Table 1 The two postremission treatment regimes
From the diagnosis of APL to the end of postremission treatment, the patients did not receive any treatment targeting psoriasis.
Definitions
HCR was defined as the presence of all of the following: no clinical evidence of APL, platelet count 100 × 109/l or greater in the blood, no blasts or promyelocytes in the blood, and less than 5% blasts in the bone marrow. Overall survival (OS) and event-free survival (EFS) were defined as previously described.Citation5 The psoriasis area and severity index (PASI) was evaluated according to the extent of involvement and the degree of erythema, scaling, and induration of psoriatic plaques in the head, trunk, arms, and legs. The score increased from 0 to 72 as the disease worsened, where 0 indicates the absence of lesions. A PASI score below 10 defines psoriasis as mild, between 10 and 20 as moderate, and above 20 as severe. The percent PASI reduction was calculated by
Statistical methods
The SPSS 15.0 software was used to analyze the data. The Kaplan–Meier method was used to develop the survival curves, and the log-rank test was used to compare the survival of groups. The survival estimates were reported as ±1 standard error. All tests were two-sided, and a P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The clinical features and treatment results of psoriasis and APL of the 17 patients are shown in Tables and . Ten of the 17 patients were males, and the patients' ages ranged from 15 to 58 years (median, 41 years) but most patients were middle-aged. Sixteen patients had a past history of psoriasis and the median duration of psoriasis was 9 years (range, 2–27 years); the other patient (Patient 11) had a new onset of psoriasis during APL treatment. Three patients had suffered from psoriasis for 20 years or more. Sixteen patients had psoriasis vulgaris, and one patient (Patient 17) had psoriasis arthropathica.
Table 2 History of psoriasis and therapeutic effect of ATO on psoriasis in the 17 patients with psoriasis-associated APL
Table 3 The baseline clinical manifestations of APL and treatment outcomes of the 17 patients with psoriasis-associated APL
The main presenting clinical manifestations included anemia (15 patients, 88%), bleeding (13 patients, 76%), and fever (8 patients, 47%). The median white blood cell count was 1.14 × 109/l (range, 0.27–28 × 109/l). Two patients (Patients 5 and 7) had hepatitis B virus infection accompanied by mild hepatic dysfunction. One patient (Patient 3) had mild renal dysfunction. Among the 10 patients who had received cytogenetic analyses, three (Patients 11, 12, and 14; 30%) had nonrecurrent chromosomal abnormalities.
Upon the occurrence of APL, of the 16 patients with a history of psoriasis, two (Patients 14 and 15) did not have skin lesions, and the other 14 patients had different degrees of psoriatic skin lesions. According to the PASI scores, six patients, six patients, and two patients had mild, moderate, and severe skin lesions, respectively. Patient 17 also exhibited a right distal interphalangeal joint deformity but had no symptoms of arthritis. At the onset of APL, none of the 14 patients had significant aggravation or relief of psoriasis symptoms.
Among the 16 patients with a history of psoriasis, one patient (Patient 8) did not receive any drug treatment, whereas the remaining 15 patients all received multiple drugs to treat psoriasis. Four patients had a definite history of taking bimolane (dose and duration unknown), and another patient (Patient 17) received methotrexate for 3 years (5 mg/week) and PUVA (psoralen–UV-A) twice. Among the three patients with nonrecurrent chromosomal abnormalities, Patient 11 had no history of psoriasis, and neither Patient 14 nor Patient 12 received bimolane treatment.
Induction therapy
Among the 17 patients, 15 (88%) achieved HCR (Table ). The median duration of induction therapy was 36 days (range: 26–50 days). The other two patients (Patients 3 and 16) died of cerebral hemorrhage on day 5 and day 17, respectively. They both had suffered from psoriasis for 20 years. The three patients with nonrecurrent chromosomal abnormalities all achieved HCR.
During the process of induction therapy, of the 14 patients who had a history of psoriasis and achieved a HCR, two (Patients 14 and 15) had no recurrence of psoriasis; one patient (Patient 8) did not exhibit significant changes in the psoriasis symptoms, and one patient (Patient 10) had aggravated psoriasis symptoms. The remaining 10 patients (10 of 12, 83%) experienced different degrees of relief of psoriasis symptoms; the percentage of PASI reduction was >90% in six patients, between 61 and 90% in three patients, and between 31 and 60% in one patient (Table ).
Postremission
The 15 patients who achieved HCR all received postremission therapy for 3–4 years. The side effects during the ATO treatment period included mild neutropenia (15 of 15), skin rashes (1 of 15), and mild hepatic dysfunction (1 of 15, not the two hepatitis B patients).
Among the 10 patients who received the B treatment regimen, three patients received a relatively high dose of cytarabine (2000 mg/m2; Table ); the DA treatment induced psoriasis in two of these three patients. Patient 11, who did not have a history of psoriasis, and Patient 14, who was in remission from psoriasis, both immediately had psoriatic skin lesions after receiving the first DA regimen, and psoriatic skin lesions gradually diffused to the whole body within 10 days (during the agranulocytosis period). During the next two cycles of DA, the psoriasis symptoms in these two patients continued to be aggravated; when the disease was the most serious, the PASI reached 4.5 and 39.6, respectively; neither patient was given anti-psoriasis treatment. The psoriatic skin lesions in the other eight patients during the DA treatment period did not exhibit significant changes.
All 15 patients received ATO postremission therapy. Patient 15 was still in the psoriasis remission period during the postremission therapy (Table ). Patient 8 still did not exhibit significant changes during the postremission therapy. Patient 10 still experienced continuing aggravation of the psoriasis symptoms during the ATO postremission therapy and the PASI reached its maximum at the last follow-up (12.6; percentage of PASI reduction was −1475%), and he was not given anti-psoriasis treatment. The other 12 patients (12 of 14, 85.7%) exhibited gradual improvement of their psoriasis symptoms during the ATO postremission therapy until the symptoms finally completely disappeared. However, during the intermittent period of ATO treatment, there was still mild recurrence of psoriasis symptoms with the presentation of mild skin redness or scaling.
Outcomes
The follow-up information was updated in August 2014. No patients were lost to follow-up (Table ). Among the 15 patients who attained HCR, two (Patients 5 and 9) underwent hematological relapse at 5 or 26 months after the achievement of HCR. Patient 5 attained a second HCR following induction with DA plus ATO and has remained in the second HCR for 26 months. Patient 9 had personally requested to discontinue postremission therapy 6 months before relapse occurred and died in relapse. The two patients had had psoriasis for 9 and 27 years, respectively, by the time they were newly diagnosed with APL. The other 13 patients remained in the first HCR. The three patients with nonrecurrent chromosomal abnormalities (Patients 11, 12, and 14) were in the first HCR for 22, 18, and 12 months, respectively.
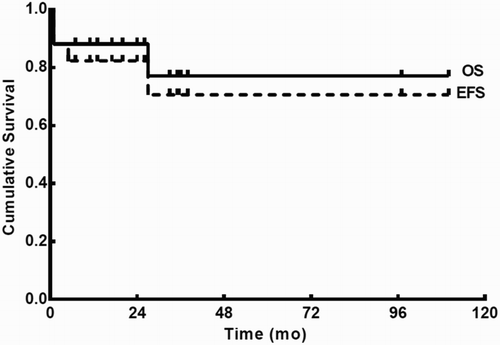
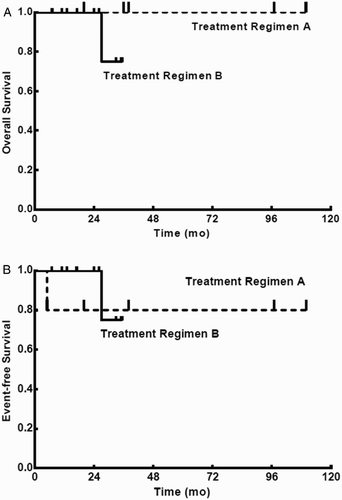
With a median follow-up of 27 months (range, 7–110 months), the 3-year estimates of OS were 77.2% ± 12.4% for all patients (Fig. ), 100% for patients who received the A treatment regimen, and 75% ± 21.7% for those receiving the B treatment regimen (P > 0.05; Fig. A); the 3-year estimates of EFS were 70.6% ± 13.5% for all patients (Fig. ), 80% ± 17.9% for patients who received the A treatment regimen, and 75% ± 21.7% for those receiving the B treatment regimen (P > 0.05; Fig. B).
Figure 2 Kaplan–Meier product limit estimate of OS (A) and EFS (B) between patients receiving treatment regimen A (n = 5) and treatment regimen B (n = 10).
Regarding the 14 survivors, at the last follow-up visit, only two patients (Patients 1 and 2) had completed the postremission treatment. Their psoriasis symptoms were not significantly aggravated after ATO withdrawal, and when there was mild recurrence, the patients self-applied topical treatments.
Through the final follow-up, no chronic arsenicosisCitation6 or secondary malignancy was observed.
Discussion
The present study revealed that patients with psoriasis exhibited a higher susceptibility to APL. Patients with psoriasis-associated APL in our hospital accounted for 8.3% of the total patients diagnosed with de novo APL during the same period, which was significantly higher than the 1–3% incidence of psoriasis in the general population. StudiesCitation1,Citation3 also showed that regarding de novo AML secondary to psoriasis, APL accounted for 55–57%, which was significantly higher than the APL incidence among patients with AML (about 10%). But almost all reports related to psoriasis-associated APL came from China,Citation1–Citation4 it remains to be seen whether the association between APL and psoriasis can be confirmed in different ethnic groups.
The fact that patients who never received any drug treatment were still more susceptible to APLCitation1 suggested that the genetic background played a certain role in the development of psoriasis-associated APL. However, familial cases of psoriasis-associated APL were rare and related fundamental researches were absent.
Drug factors play important roles in the susceptibility of psoriasis patients to APL. It is reported that of patients with psoriasis-associated APL, 40% had a definite history of bimolane treatment.Citation1 Bimolane is an anti-tumor drug that can inhibit DNA synthesis. It could effectively inhibit the excessive proliferation of the involved epidermal cells in psoriasis patients thus alleviate the symptoms of psoriasis. In China, bimolane was applied extensively to treat psoriasis from 1982 until October 2002 when the drug was banned because they could induce secondary leukemias. Ten of the patients in our study had a psoriasis onset before October 2002, of whom four patients had a definite history of bimolane treatment. Frantz et al.Citation7 found that bimolane had significant inhibitory effects on topoisomerase II. Studies have revealed that bimolane exhibited forceful mutagenicity, which could induce chromosomal breakage and aberrations in cultured human lymphocytesCitation8,Citation9 and mouse bone marrow cells.Citation10 These features make bimolane a potential genotoxic, cytotoxic, and leukemogenic agent.Citation9 Wang et al.Citation1 confirmed that nonrecurrent chromosomal abnormalities were more frequent in patients with psoriasis-associated APL and a history of bimolane treatment than in those without bimolane treatment.
One patient (Patient 17) in our study had a history of methotrexate and PUVA treatment. Lakhani et al.Citation11 also reported a patient who developed APL after treated for psoriasis with methotrexate and hydroxyurea followed by razoxane. Whereas there are no other reports showing that treating psoriasis with methotrexate and/or PUVA induced APL.
The clinical manifestations of psoriasis-associated APL were similar to those of general APL and the onset of APL did not aggravate the psoriasis symptoms. An ATO-based regimen for remission induction yielded a high complete remission rate in psoriasis-associated APL, which were comparable with that produced by use of the combination of ATRA and chemotherapy in general APL.Citation12 In this study, the OS of patients with psoriasis-associated APL was somewhat low. In fact, only one patient (Patient 9) died of relapse, which was most likely brought on by the self-termination of postremission therapy. It is nothing unusual for studies with small sample sizes, one death may bring about a considerable drop in the survival curve.
Wang et al.Citation1 reported that more than 10-year course of psoriasis and nonrecurrent chromosomal abnormalities were poor prognostic factors for HCR and relapse-free survival, respectively. Among the three patients who had suffered from psoriasis for 20 years or more in our study, two died during induction therapy and the other died in the first relapse 27 months after achieving HCR. Whereas the three patients with nonrecurrent chromosomal abnormalities all obtained durable remission without relapse.
In addition to being highly effective for APL, ATO could also significantly improve the psoriasis symptoms. In ancient China and Europe, ATO was used extensively for treating psoriasis. However, the protracted use of arsenic in psoriasis led to chronic arsenicosis and even to secondary malignancy. Therefore, its use was halted.Citation13 Currently, arsenic has again attracted people's attention, mainly because of the unique efficacy of ATO for treating APL. However, due to the observed risks of long-term arsenic exposure, protracted use of ATO is still difficult to accept. It is our hospital who first applied ATO for treating APL in the 1970s and we have confirmed that ATO-based regimen which lasts 3–4 years is highly safe, even for patients with mild hepatic or renal dysfunction.Citation5,Citation14 The completely opposite results might be due to the following reasons. First, an entirely different ATO administration method was used. Previously, ATO was administered through topical or oral administration, or subcutaneous injection; whereas currently, ATO was administered by intravenous drip. Second, the maximum daily dose of ATO was limited to 10 mg in our regimen. This really matters. Any substance, even including essential nutritional substance for the human body, would become a ‘poison’ if its intake exceeds a certain amount. A higher daily dose of ATO could often cause severe toxicities.Citation15–Citation17 ATO could be a new treatment option for psoriasis patients who are resistant to all the common anti-psoriasis treatments.
It is noted that Patient 11, who had no history of psoriasis and had a new onset of psoriasis during postremission therapy, was also included in this study. Similar to those in other patients, the occurrence of psoriasis did not cause relapse of APL and subsequent treatments with ATO were effective for psoriasis.
It has been reported that DA induction therapy could improve psoriasis symptoms in two patients with psoriasis-associated M4 or psoriasis-associated M2.Citation18,Citation19 In this study, during postremission therapy, DA treatment did not reduce psoriasis severity, it even induced psoriatic skin lesion in a patient who had been in remission from psoriasis for years and in the patient without a psoriasis history. The different subtypes of AML, the distinct stages of AML (before HCR/after HCR), and the various doses of cytarabine all might be possible reasons for the disparate responses to DA treatment by different psoriasis patients.
Conclusion
This study revealed that psoriasis patients are at high risk for APL. The increased susceptibility to APL in psoriasis patients is most likely associated with the genetic background and bimolane treatment. The clinical characteristics and treatment outcomes of psoriasis-associated APL are similar to those of general APL. ATO-based therapy is especially suitable for patients with psoriasis-associated APL. Our study also brings a new treatment option for psoriasis.
Disclaimer statements
Contributors Y.Z. and F.G. contributed equally to this work. J.Z. designed the study. J.L., S.L., L.L., X.L., S.W., C.L., and Y.S. participated in the diagnosis, treatment and follow-up of the patients. J.H., P.W., H.L., M.X., and F.C. reviewed the medical record, arranged the data and searched the literature. F.G. analyzed the data and made the figures. Y.Z. and F.G. wrote the manuscript. All authors read and approved the final manuscript.
Funding None.
Conflicts of interest None.
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the First Affiliated Hospital of Harbin Medical University's Medical Ethics Committee.
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (No. 81270589), Natural Science Foundation of Heilongjiang Province (No. D201010), and the National High Technology Research and Development Program of China (863 Program, No. 2012AA020903).
References
- Wang Y, Mi Y, Li D, Xue Y, Bian S, Wang J. Acute leukemia association with psoriasis: a report on 100 patients from a single center in China. Am J Hematol. 2010;85:378–9. doi: 10.1002/ajh.21729
- He Y, Lu X, Qiu J, Zhu T. Severe vulgaris psoriatic patients with acute myelogenous leukaemia and resolution after allogeneic bone marrow transplantation/peripheral blood stem cell transplantation. Chinese Medical Journal. 2005;118:861–5.
- Xue Y, Lu D, Guo Y, Lin B. Specific chromosome translocations and therapy-related leukemia induced by bimolane therapy for psoriasis. Leukemia Res. 1992;16:1113–23. doi: 10.1016/0145-2126(92)90050-H
- Au W, Yeung C. Acute promyelocytic leukemia in patients with severe psoriasis vulgaris. Leukemia Res. 2009;33:e189–90. doi: 10.1016/j.leukres.2009.05.001
- Zhou J, Zhang Y, Li J, Li X, Hou J, Zhao Y, et al. Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood. 2010;115:1697–702. doi: 10.1182/blood-2009-07-230805
- Guha Mazumder DN. Chronic arsenic toxicity and human health. Indian J Med Res. 2008;128:436–47.
- Frantz CE, Smith H, Eades DM, Grosovsky AJ, Eastmond DA. Bimolane: in vitro inhibitor of human topoisomerase II. Cancer Lett. 1997;120:135–40. doi: 10.1016/S0304-3835(97)00303-0
- Roy SK, Eastmond DA. Bimolane induces multiple types of chromosomal aberrations in human lymphocytes in vitro. Mutat Res. 2011;726:181–7. doi: 10.1016/j.mrgentox.2011.09.006
- Vuong MC, Hasegawa LS, Eastmond DA. A comparative study of the cytotoxic and genotoxic effects of ICRF-154 and bimolane, two catalytic inhibitors of topoisomerase II. Mutat Res. 2013;750:63–71. doi: 10.1016/j.mrgentox.2012.09.005
- Chen SQ, Xue KX, Wu JZ, Ma GJ. Mutagenic effects of bimolane. Zhongguo Yao Li Xue Bao. 1995;16:531–3.
- Lakhani S, Davidson RN, Hiwaizi F, Marsden RA. Razoxane and leukemia. Lancet. 1984;2:288–9. doi: 10.1016/S0140-6736(84)90330-1
- Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S., et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–6. doi: 10.1182/blood-2009-07-233387
- O'Daly J, editor. Psoriasis – a systemic disease. Rijeka, Croatia: InTech Publishing; 2012. p. 216.
- Zhang Y, Zhang Z, Li J, Li L, Han X, Han L., et al. Long-term efficacy and safety of arsenic trioxide for first-line treatment of elderly patients with newly diagnosed acute promyelocytic leukemia. Cancer. 2013;119:115–25. doi: 10.1002/cncr.27650
- Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K., et al. Arsenic trioxide therapy for relapsed or refractory Japanese patients with acute promyelocytic leukemia: need for careful electrocardiogram monitoring. Leukemia. 2002;16:617–22. doi: 10.1038/sj.leu.2402426
- Westervelt P, Brown R, Adkins D, Khoury H, Curtin P, Hurd D., et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. 2001;98:266–71. doi: 10.1182/blood.V98.2.266
- Unnikrishnan D, Dutcher J, Varshneya N, Lucariello R, Api M, Garl S., et al. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood. 2001;97:1514–6. doi: 10.1182/blood.V97.5.1514
- Kohn D, Flatau E, Daher O, Zucjerman F. Treatment of psoriasis with daunorubicin and cytarabine. Arch Dermatol. 1980;116:1101–2. doi: 10.1001/archderm.1980.01640340011006
- Paslin D. Psoriasis without neutrophils. Int J Dermatol. 1990;29:37–40. doi: 10.1111/j.1365-4362.1990.tb03753.x