Abstract
Objectives: This is a feasibility study to evaluate whether simultaneous administration of low doses of imatinib and nilotinib in chronic-phase chronic myeloid leukaemia (CP-CML) patients has the potential for transcript elimination after failure to imatinib.
Methods: Ten patients were enrolled; eight had cytogenetic relapse and two had confirmed loss of major molecular response (MMR). At baseline, BCR-ABL kinase domain mutation was detected in four patients.
Results: After 6 months of therapy, major cytogenetic response, complete cytogenetic response, and MMR were achieved in seven, four, and four patients, respectively. Grade 4 thrombocytopenia developed in one patient, and grade 1 skin rash in four.
Discussion and conclusion: These results suggest that imatinib might have inhibitory effects on the clearance of nilotinib, increasing its efficacy. This dual therapy was well tolerated and resulted in improvement of cytogenetic and molecular responses in patients with CP-CML after failure to imatinib. ClinicalTrials.gov registration number: NCT01819389.
Introduction
Tyrosine kinase inhibitors (TKIs) have improved the outcome of patients with chronic myeloid leukaemia (CML) over the last decade. Imatinib, the first TKI licensed for CML, has been shown to be highly effective, it has a well-known side-effect profile with no reports of significant late side effects. Unfortunately, 15–20% of patients with chronic-phase (CP) CML show primary or secondary resistance or failure to imatinib.Citation1–Citation4 Switching to any of the second-generation TKI is the most recommended strategy after imatinib resistance.Citation5 Nilotinib, dasatinib, and bosutinib have shown superiority over imatinib in newly diagnosed CP CML patients, particularly in the depth and speed of response. Despite encouraging results obtained with these newer TKIs, current data demonstrate that use of a single TKI is insufficient to completely eradicate the disease in some patients that eventually fail to achieve or maintain an optimal response, requiring alternative treatment.Citation6,Citation7 The persistence of Philadelphia (Ph)-positive stem cells, which are resistant to TKIs, is believed to be the reason for this failure.Citation8–Citation10
Preclinical studies suggest that use of more than one TKI is associated with a synergistic effect. Nilotinib is a BCR-ABL1 inhibitor more potent and selective than imatinib. The combined use of imatinib and nilotinib shows no antagonistic effect and has demonstrated positive evidence against many imatinib-sensitive and -resistant cell-lines.Citation11–Citation13 Like imatinib, nilotinib inhibits BCR-ABL by binding to the inactive Abl kinase conformation; however, these drugs differ in their mechanism of cellular transport.Citation12,Citation13 The benefit of combined therapy appears to be mediated through an increase in the intracellular concentration of nilotinib.Citation11–Citation13 White et al . suggested that imatinib inhibition of protein-mediated efflux may be the cause of increased intracellular uptake and retention for nilotinib.Citation12 In one study, a more profound suppression of resistant clone outgrowth was observed using TKI combinations compared with single agents.Citation14 Furthermore, the combination of imatinib and nilotinib has been reported in the treatment of patients with gastrointestinal stromal tumours (GISTs),Citation15 and in at least two CML patients, without increased toxicity.Citation16,Citation17 Based on this evidence we performed a study combining these two TKIs. Our objective was to determine whether simultaneous use of low-dose imatinib and low-dose nilotinib has the potential for improving cytogenetic and molecular responses in CP-CML patients after failure to imatinib monotherapy. An additional objective was to evaluate the tolerability of this combination.
Patients and methods
From January 2013 to July 2013, a total of 10 patients with CP-CML who presented failure to imatinib were included. The Ethics Committee of the School of Medicine and University Hospital of the Universidad Autónoma de Nuevo León approved this single-arm study. The eligibility criteria were as follows: adults with a diagnosis of CP-CML who were treated with imatinib and developed secondary resistance to the drug, normal renal, hepatic, and cardiac function, and adequate performance status (World Health Organization performance score 0 or 1). For this analysis, failure was defined as the loss of complete cytogenetic response (CCyR), called cytogenetic relapse, or the confirmed loss of a major molecular response (MMR). Patients with imatinib intolerance, non-adherence, and primary resistance were excluded. Patients gave written informed consent in accordance with the Declaration of Helsinki and institutional guidelines. This study is registered at ClinicalTrials.gov identifier: NCT01819389.
Patients received imatinib 200 mg and nilotinib 300 mg daily for 6 months. Imatinib was taken with breakfast, whereas nilotinib was taken in the afternoon. Patients fasted for at least 2 hours prior to the dose, as well as 1 hour after taking nilotinib. Nilotinib was discontinued in the case of disease progression, unacceptable toxicity, or withdrawal of consent.
Cytogenetic response was assessed using conventional cytogenetic analysis performed on bone marrow cells with the G-banding technique. At least 20 metaphases were analysed to determine the presence of Ph-positive metaphases.
Molecular response was assessed using a real-time polymerase chain reaction (RT-PCR), expressed as the BCR-ABL/ABL ratio (international scale). Sequencing of the Abl kinase domain was assessed through mutational analysis. Complete blood count and biochemistries were obtained at each study visit: biweekly the first 2 months and monthly thereafter. Cytogenetic analysis was performed at baseline and at 6 months. Mutational analysis was done only at baseline. Molecular response was assessed at baseline, 3, and 6 months. Standard definitions of cytogenetic and molecular responses were usedCitation5: CCyR, 0% Ph-positive metaphase, partial cytogenetic response (PCyR), 1–35%; minor cytogenetic response (mCyR), 35–65%; and minimal cytogenetic response (miCyR), 66–95%. Major cytogenetic responses (MCyRs) include CCyR and PCyR. For MMR, a BCR-ABL expression level of ≤0.1% is equivalent to a ≥3 log reduction from the standardized baseline. It is important to note that all samples were analysed in a central reference laboratory. Safety was assessed at each study visit according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0
Results
Ten patients with imatinib failure in CP-CML were enrolled at our institution. Eight patients had cytogenetic relapse and two had confirmed loss of MMR without cytogenetic relapse. Median age was 38 years (range, 27–68). Median duration of CML was 5.4 years (range, 2.7–12.4) and duration of imatinib therapy was 5 years (range, 2.7–9.5) with a median dose of 400 mg (range, 400–800 mg). All patients had a complete hematologic response at baseline. Nine patients completed 6 months of combined therapy (imatinib 200 mg plus nilotinib 300 mg) without interruption; one patient discontinued therapy at month 3 due to an adverse event and was considered a non-responder.
Response
MCyR was achieved in seven patients, with CCyR occurring in four (50%) of the eight patients with cytogenetic relapse. Among the four patients who had more than 95% Ph+ cells at baseline, PCyR was achieved in three (75%). One patient with miCyR, one with mCyR, and two with PCyR at baseline achieved CCyR (Table ).
Table 1 Basal characteristics and results
MMR was achieved in 4 (40%) out of 10 patients. Two of the four patients that achieved CCyR obtained MMR at month 6. The two patients with loss of MMR without cytogenetic relapse at baseline achieved MMR, one patient at 3 months and the other at 6 months.
Mutations analysis
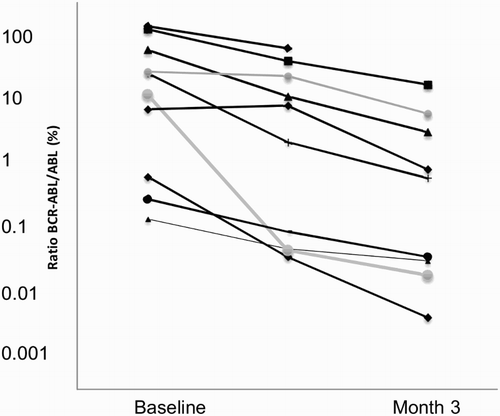
BCR-ABL kinase domain mutations were detected in four patients at the start of the study; all mutations were associated with clinical resistance to imatinib, but not to nilotinib.Citation18 The four patients with mutations had cytogenetic relapses at baseline. At month 6 of combination therapy, CCyR and PCyR were achieved in one and two patients, respectively. Kinetics analysis of transcript levels of the 10 patients from baseline to month 6 indicates an ongoing reduction of BCR/ABL transcript levels (Fig. ).
Adverse events
Four patients experienced a grade 1 skin rash – two patients responded with antihistamines and two with corticosteroid concomitant therapy. Only one patient discontinued combination therapy due to grade 4 thrombocytopenia. Imatinib (600 mg) was resumed 3 weeks after withdrawal with a normal platelet count; at the last follow-up visit, this patient had progressed to an accelerated phase.
Discussion
Imatinib revolutionized CML treatment because it was the first single drug to specifically target the BCR-ABL tyrosine kinase protein. However, new more potent drugs were developed and they are now used as monotherapy. Nonetheless, there are case reports using imatinib plus nilotinib in CML. On the other hand, the association of TKIs has been effective in patients suffering from a GIST suggesting that imatinib might have inhibitory effects on the clearance of nilotinib.Citation15–Citation17
The present study is the first trial to examine a TKI combination in CP-CML patients. Our results show that this novel combination is feasible, and well tolerated. In our patients rash was the most common adverse event. Only one patient discontinued the study due to grade 4 thrombocytopenia.
In a recent study, patients in CCyR with detectable BCR-ABL1 after >2 years on imatinib were randomized to nilotinib (400 mg twice daily) or continued imatinib. By 6 months, MR 4.5 BCR-ABL1 was achieved in 12 and 5% of patients in the nilotinib and imatinib arms, respectively.Citation18 In our study, using a less-sensitive PCR, 40% of patients achieved MMR at 6 months. With this combination of TKIs 9 (90%) patients improved their response, MCyR, CCyR, and MMR were achieved in seven, four, and four patients, respectively. In studies of CP-CML, patients with imatinib failure and/or intolerance who received a second-generation TKI MCyR was achieved in 31–59% and CCyR in 23–45% of patients after 6–8 months.Citation19–Citation21
Rates of MMR previously reported with second-generation TKIs as second-line therapy range from 28 to 41% at 12–24 months.Citation21–Citation23 Despite the short follow-up of this trial, the kinetics analysis of transcript levels from baseline to month 6 indicates an ongoing reduction of BCR/ABL expression levels (Fig. ). We hypothesized that the combination of these drugs may improve molecular response, but it needs further testing. Although there is no doubt that newer generation TKIs are more potent, imatinib remains a very effective and safe drug.Citation2,Citation5 It is possible that TKI combined therapy could improve the response rate achieved with imatinib monotherapy. In the treatment of CML, it is important to consider others factors, such as cost. Low-dose imatinib combined with low-dose nilotinib could provide an affordable alternative for CML patients after imatinib failure.
Conclusions
The conclusions from our study are limited but important due to the potential benefit of this approach. The combination of imatinib and nilotinib is feasible. Further investigation of the synergistic effect of imatinib and nilotinib combination therapy in patients with CML is warranted.
Disclaimer statements
Contributors D.G.-A. created and designed the research, and gave final approval of the manuscript for publication. R.S.-V. and C.H.G.-A. performed the research. O.G.C.-R. collected and analysed the data. L.T.-A., M.A.H.R, A.V.d.L., and J.C.J-P. drafted the paper. All authors approved the manuscript.
Funding None.
Conflicts of interest All authors declare no conflicts of interest.
Ethics approval The Ethics Committee of the School of Medicine and University Hospital of the Universidad Autónoma de Nuevo León approved this single-arm study.
Acknowledgements
The authors would like to thank the patients who participated in this study. Imatinib was provided by the Glivec International Patient Assistance Program (GIPAP) system, administered by the Max Foundation (www.maxaid.org). The Hematology Service provided nilotinib. We acknowledge Novartis Mexico for the financial support for the cytogenetic, molecular, and mutational analysis of our patients.
References
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457
- Deininger MW, O'Brien SG, Guilhot F, Goldman JM, Hochhaus, A, Hughes, TP, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH annual meeting abstracts. Blood. 2009;114(22):1126.
- Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758–65. doi: 10.1182/blood-2010-03-273979
- Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84. doi: 10.1182/blood-2013-05-501569
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614
- Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006; 107(11):4532–9. doi: 10.1182/blood-2005-07-2947
- Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109(9):4016–9. doi: 10.1182/blood-2006-11-057521
- Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–24. doi: 10.1038/leu.2010.185
- Weisberg E, Catley L, Wright RD, Moreno D, Banerji L, Ray A, et al. Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood. 2007;109(5):2112–20. doi: 10.1182/blood-2006-06-026377
- White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108(2):697–704. doi: 10.1182/blood-2005-11-4687
- White DL, Saunders VA, Quinn SR, Manley PW, Hughes TP. Imatinib increases the intracellular concentration of nilotinib, which may explain the observed synergy between these drugs. Blood. 2007;109(8):3609–10. doi: 10.1182/blood-2006-11-058032
- Bradeen HA, Eide CA, O'Hare T, Johnson KJ, Willis SG, Lee FY, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006;108(7):2332–8. doi: 10.1182/blood-2006-02-004580
- Demetri GD, Casali PG, Blay JY, von Mehren M, Morgan JA, Bertulli R, et al. A phase I study of single-agent nilotinib or in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Clin Can Res. 2009;15(18):5910–6. doi: 10.1158/1078-0432.CCR-09-0542
- Gomez-Almaguer D, Tarin-Arzaga L, Cantu-Rodriguez O, Ceballos-Lopez A. More about imatinib and nilotinib combination therapy in chronic myeloid leukemia. Acta Haematol. 2013;129(1):18–9. doi: 10.1159/000342455
- Zhu GR, Ji O, Ji JM, Zhang YC, Wu Y, Yu H, et al. Combining nilotinib and imatinib improves the outcome of imatinib-resistant blast phase CML. Acta Haematol. 2012;127(3):152–5. doi: 10.1159/000333107
- Hughes TP, Lipton JH, Spector N, Cervantes F, Pasquini R, Clementino NC, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014;124(5):729–36. doi: 10.1182/blood-2013-12-544015
- Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–6. doi: 10.1182/blood-2007-03-080689
- Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–12. doi: 10.1200/JCO.2007.14.9260
- Cortes JE, Kantarjian HM, Brummendorf TH, Kim DW, Turkina AG, Shen ZX, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17):4567–76. doi: 10.1182/blood-2011-05-355594
- Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27(1):107–12. doi: 10.1038/leu.2012.181
- Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–6. doi: 10.1038/leu.2008.84