Abstract
Objectives: Our aim was to retrospectively investigate the real-world outcome and healthcare costs associated with the treatment of patients with relapsed or refractory multiple myeloma (RRMM) in a Chinese single center.
Methods: A retrospective study was conducted for 93 patients between January 2008 and December 2013 in a Chinese hematology department. Total monthly costs attributable to each cost component were described across all regimens and for bortezomib-based treatment regimens.
Results: Mean total cost per patient-month ($1139.85) varied depending on the sequence of therapy (range: mean $51.63–$6600.96). Drugs and hospital visit were the most and the least consumed resource (65.48% and 2.87%, respectively). Mean total monthly costs were $2071.96 (range: $679.73–$6600.96) and $551.14 (range: $51.63–$1698.59) for patients receiving bortezomib and patients not receiving bortezomib, respectively. Differences between the two groups were significant for drugs; drugs costs were higher for patients treated with bortezomib. Kaplan–Meier curves showed a longer overall survival (mean [median] 31.43 [25] vs. 21.93 [18] months) for patients treated with bortezomib.
Conclusion: Real-world costs during treatment of RRMM varied greatly. Total costs during bortezomib-based regimens are significantly higher compared with non-bortezomib regimens. Further multi-center studies are needed to assess the cost-effectiveness of bortezomib for the treatment of RRMM in China.
Introduction
Multiple myeloma (MM) is a B cell lymphoproliferative disorder that remains an incurable disease and typically follows a relapsing course. The most common symptoms of MM are fatigue, anemia, bone pain, hypercalcemia, renal dysfunction, and recurrent infections.Citation1 The ethnic variation in the incidence of multiple myeloma is well known, with a lower incidence reported in Asian countries than in western countries. In China, MM is the third most common hematologic malignancy after leukemia and non-Hodgkin's lymphoma and the annual incidence of MM is less than 1 per 100 000 people.Citation2
Prognosis for myeloma is severe with a median survival of approximately 3 years with conventional treatment. With the using of high-dose chemotherapy plus hematopoietic stem cell transplantation as frontline therapy, a significant improvement of long-term survival had been achieved in younger patients. In the last decade, the introduction of novel targeted agents has dramatically improved overall survival in MM, such as thalidomide, lenalidomide, and bortezomib.Citation1 However, their substantial cost restricts their widespread use, especially in health resource-limited regions. Economic evaluations are increasingly required to estimate the costs and outcomes of therapies in order for these to be adequately financed.Citation3
Previous economic evaluations were based on cost analyses, during which synthesizing data and expert opinions instead of patient-level data were employed to estimate resource use.Citation4–Citation6 High costs of novel agents increasingly put pressure on limited healthcare budgets. Many decision makers are interested in the ‘real-world’ costs and cost-effectiveness of expensive drugs. However, very few studies have been conducted using real-world cost data on MM, and there is still no evidence of this kind in China. Therefore, the primary objective of this study was to retrospectively investigate the real-world outcome and health care costs associated with the treatment of patients with relapsed or refractory multiple myeloma (RRMM) in a Chinese single center.
Patients and methods
Data collection
This was a single-center retrospective observational study. A total of 93 patients were recruited because they were treated for RRMM in daily clinical practice between January 2008 and December 2013. Patients were at least 18 years of age and had either relapsed following or were refractory to first-line treatment; had a follow-up of at least 12 months in the center. Detailed data were retrospectively collected using medical charts and electronic records from the time of first relapsed/refractory disease until their date of death or end of the study period. Data were collected with the following items: baseline patient characteristics, drugs and dosages, treatment used for RRMM at each line, adverse effects, and resource use. This study was approved in accordance with the principles of the ethics committee in Wuxi People's Hospital.
Cost analysis
Cost analysis was performed from the perspective of the Chinese healthcare system. Direct medical hospital-based costs, such as treatment-related medicines, radiological and laboratory examinations, management of serious adverse events (SAEs) and concomitant treatments, hospital stays were included. All monetary values were expressed in US dollars, year 2012 values (1 USD = 6.3125 RMB). All the unit costs of the health resources were estimated using data from the local health systemCitation7 or the National Development and Reform Commission (NDRC) of China.Citation8 Costs and resources were aggregated per patient according to the line of treatment and divided by the total number of months spent in that line, respectively.
Statistical analysis
Continuous variables were summarized as mean, standard deviation, median, and extreme values; categorical data as absolutes and percentages. Comparisons of the use of resources and costs were carried out by Mann–Whitney test and Fisher's exact test. Overall survival (OS) was measured from start of relapsed/refractory of treatment to date of death or last follow-up. Survival curves were plotted using the Kaplan–Meier method, with differences assessed with the log-rank test. Statistical analyses were performed using SPSS statistical software (version 19.0). P-values < 0.05 were considered significant.
Results
Patient characteristics
The study included 93 patients with RRMM; 36 received bortezomib and 57 did not receive bortezomib. Baseline characteristics of all patients are shown in Table . The median age was 65 years (range, 40–79 years) at start of relapsed/refractory. There were more men (n = 51, 55%) and more patients (n = 47, 51%) identified with IgG myeloma in the study. The common disease stage was IIIA. At the time of data collection (31 December 2013), 44 patients were still alive. The median survival from diagnosis to last follow-up for all patients was 39 (range, 12–103 months) months. Significant differences between patients treated and not treated with bortezomib (Table ) were observed for type of myeloma (P = 0.014) and World Health Organization performance status (P = 0.017).
Table 1 Patients’ characteristics at start of relapsed/refractory treatment
Resource consumption
Drugs and hospital visit were the most and the least consumed resource (65.48 and 2.87%, respectively) (Table ). Chemotherapy drug prescriptions are presented in Table . A total of 14 different chemotherapy drugs were recorded. A total of 93, 37, and 16 patients were received second, third, and fourth line treatment, respectively. A combination of treatments was common practice; VAD (vincristine/adriamycin/dexamethasone) was most commonly administered in second line (44.09%). Bortezomib-based regimens were administered most often in third and fourth lines (59.46 and 56.25%, respectively). Thirty-six patients received bortezomib at least once and 15 of them during two different lines. Bortezomib was used in combination that is with dexamethasone (5.56%), thalidomide plus dexamethasone (41.67%), pegylated liposomal doxorubicin (33.33%) or doxorubicin plus dexamethasone (8.33%), Melphalan plus prednisone (8.33%), lenalidomide plus dexamethasone (2.78%).
Table 2 Unit costs of drugs and treatments received by treatment line (USD 2012)
Table 3 Mean monthly costs during relapsed/refractory treatment
Costs during relapsed/refractory treatment
Mean monthly costs incurred during all treatment regimens are presented in Table . Irrespective of treatment line-order, the mean total cost per patient-month was $1139.85 (range: $51.63–$6600.96) or $20128.70 per patient (range: $826.14–$130474.99). There was large variation in costs for therapy regimens within the lines as well as across lines. Mean total costs during second and third line were $904.64 per month (range: $51.63–$4819.26) or $10618.04 per patient (range: $826.14–$38220.12) and $2638.11 per month (range: $166.68–$21941.01) or $17000.44 per patient (range: $1666.82–$87764.04), respectively. Mean total costs during fourth line were $2725.58 per month (range: $596.18–$6660.58) or $16337.37 per patient (range: $1897.26–$61841.26). Mean total costs during third line and fourth line were higher compared with second line due to more frequent use of novel agents, especially bortezomib and lenalidomide. Generally, costs of therapy were drivers of increased costs across all regimens.
Costs during bortezomib-based regimens
Table describes the mean monthly costs for patients treated with bortezomib (n = 36) and patients not treated with bortezomib (n = 57). For patients treated with bortezomib, the mean total cost per patient-month was $2071.96 (range: $679.73–$6600.96) or $38772.33 per patient (range: $12914.94–$130474.99). For patients not treated with bortezomib, the mean total cost per patient-month was $551.14 (range: $51.63–$1698.59) or $8353.77 per patient (range: $826.14–$49520.8). Differences between the two treatment groups were significant for drugs, concomitant treatment and hospital stays. Costs during bortezomib-based regimens were mainly attributable to significantly higher acquisition costs of bortezomib. Approximately 74% of mean monthly costs incurred during bortezomib-based regimens were attributable to the acquisition costs of drug, compared with 45% during non-bortezomib regimens.
Mean total monthly costs were significantly lower in second, third, and fourth line during non-bortezomib regimens ($515.26, $928.33, and $810.35, respectively) compared with bortezomib-based regimens ($1963.76, $3803.87, and $4215.2, respectively) (Table ). Differences between the two groups were significant only for drugs; drugs costs were higher for patients treated with bortezomib. The discrepancy between these two formats (the lines and across all lines) was due to the variability in individual patients’ costs and length of follow-up.
Table 4 Mean monthly costs during each line treatment with bortezomib
Treatment effects
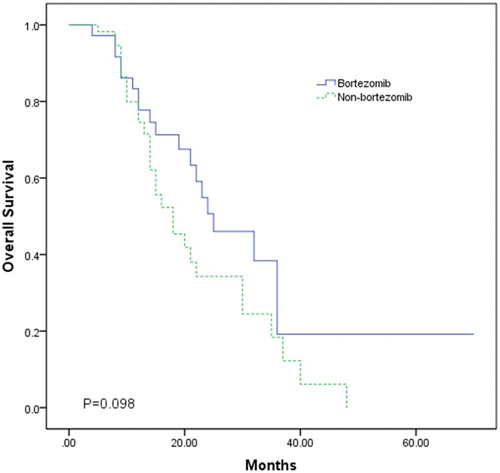
OS from start of relapsed/refractory disease was used to analyze the treatment effects. The mean follow-up duration was 21.1 ± 12.2 and 15.0 ± 9.2 months for patients treated and patients not treated with bortezomib, respectively. At the end of data collection, 17 patients treated with bortezomib and 27 patients not treated with bortezomib were still alive. Kaplan–Meier curves (Fig. ) from start of relapsed/refractory treatment showed a longer mean (31.43 vs. 21.93 months) and median (25 vs. 18 months) OS for patients receiving bortezomib (Log-rank P = 0.098; Wilcoxon P = 0.262).
Real-world cost-effectiveness
Due to great differences in baseline characteristics and extensive treatment variation, it was impossible to obtain valid and precise incremental cost-effectiveness estimates of bortezomib compared with other treatments. Only costs per month of survival were evaluated for patients treated and patients not treated with bortezomib. The costs from start of relapsed/refractory treatment for patients treated with bortezomib were $1233.61 per month of survival (total mean costs $38772.33; mean OS 31.43 months). However, for patients not treated with bortezomib, the costs were $380.93 per month of survival (total mean costs $8353.77; mean OS 21.93 months).
Discussion
In the late 1990s, the therapeutic landscape of MM has changed with the introduction and increasingly widespread use of thalidomide, lenalidomide, and bortezomib. The novel targeted therapies have significantly improved the response rates, progression-free and potentially overall survival of MM patients in both the frontline and recurrent settingsCitation9–Citation11 and have created a much broader range of options for clinicians and patients. Furthermore, associated enhanced supportive care measures have also been developed to improve MM management.Citation12 However, these multidrug regimens are more expensive, and the costs of therapy have become increasingly important to payers and patients. Not only is efficacy of therapy important but cost-effectiveness is also a critical consideration for patients and cancer treatment centers, especially in resource-limited settings such as China. Increasingly, healthcare systems are demanding the real-world costs and cost-effectiveness of treatments as part of authorizing funding and reimbursement of new agents.
Although China is a country with a relatively low incidence rate of MM, with the acceleration of the aging process, it is predicted that MM, with a rapid growth in incidence, will become one of the more significant diseases that affect people's health in China. A strategy that includes thalidomide and bortezomib was recommended in the 2008 Chinese Clinical Practice Guidelines in Multiple Myeloma.Citation13 Besides, lenalidomide was added in the 2013 guidelines.Citation14 China has a large population (1.3 billion) with differing degrees of education and a large disparity between the rich and poor. The growing cost of treatment for MM will become a serious economic burden for society, families, and patients. Constrained economic conditions result in different treatment options. Clinicians must consider costs when recommending treatment regimens. In China, the development of pharmacoeconomics is slow and conducting pharmacoeconomic research is difficultCitation15 and data on the cost of MM treatments are not available. Therefore, studying the economic evaluations in MM is important for the fields of medicine and policy.
Our study revealed that the direct medical cost of treatment for an average RRMM patient-month amounted to approximately $1139.85 (range: $51.63–$6600.96). Costs during treatment of RRMM varied greatly and were largely attributable to acquisition costs of both novel and expensive agents. In addition, costs varied also by treatment-related resource use. Total costs during bortezomib-based regimens were significantly higher compared with non-bortezomib regimens. The two treatment groups were significantly different in drugs, concomitant treatment and hospital stays. Lower costs were found in our cost analysis than those reported.Citation16–Citation21 Such differences may occur due to variation in the systems of care provision, financing and clinical practice patterns. China is a developing country, in which costs of medical services and examinations are far less expensive compared to developed countries. Although the overall cost is less in China, the expense represents a larger proportion of income.
To our knowledge, the present report was the first economic analysis evaluating the real-world outcome and health care costs of RRMM in China. Our data came from a database of real clinical cases, which likely better reflected the true medical care costs for patients. The presented results will be an important reference for Chinese health economists undertaking further economic analyses, including the use of mathematical modeling in evaluating the cost-effectiveness of treating RRMM.
There are several limitations to this study. First, this was a retrospective study at one local hospital; therefore, our results may not generalize to all hospitals. To comprehensively evaluate the cost of medical care in China, it will be necessary to collect data from more hospitals across the country. However, the geographical area and the type of setting (a typical regional university hospital) might make it partly representative for east China. Second, because the great heterogeneity and extensive treatment variation resulted in incomparable patient groups, it was impossible to develop a feasible model to obtain valid and precise incremental cost-effectiveness estimates of bortezomib compared with other treatments. Third, for economic reasons, some patients had discontinued therapy before the maximum response had been reached, especially patients receiving bortezomib, and survival may have been affected by this. The median number of cycles of bortezomib treatment was 2 (range: 1–4). Most patients only received 1 or 2 cycles. Fourth, only hospital-related resources and costs were include in our study, however, the cost analysis adopting the societal perspective and accounting for all direct and indirect costs should also be evaluated. Fifth, there was no opportunity to provide an estimate of the cost-utility of treatments, as we could not collect data on quality of life of patients with RRMM due to a retrospective research design.
In conclusion, our study shows high use of bortezomib after failure of second line treatment and a high cost associated with it. The economic evaluation of health care technologies is becoming more important in China, especially in MM for which new and expensive therapies are being introduced regularly. It will contribute to the more efficient allocation of limited healthcare resources in China. Moreover, further multi-center studies on larger samples of patients and long-term follow-up are needed.
Disclaimer statements
Contributors H.F.G. and X.Z. were the principal investigators and take primary responsibility for the paper; J.X., J.J.M., F.C., and X.F.Q. recruited the patients and collected the data; H.F.G. wrote the paper.
Funding None.
Conflicts of interest None of the authors have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Ethics approval This study was approved in accordance with the principles of the ethics committee in the Wuxi People's Hospital.
References
- Kyle RA, Rajkumar SV. Treatment of multiple myeloma. A comprehensive review. Clin Lymphoma Myeloma. 2009;9:278–88. doi: 10.3816/CLM.2009.n.056
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer incidence and Mortality and Prevalence Worldwide in 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed 31 March 2015).
- Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good research practices task force report. Value Health. 2009;12:409–18. doi: 10.1111/j.1524-4733.2008.00489.x
- Mehta J, Duff SB, Gupta S. Cost effectiveness of bortezomib in the treatment of advanced multiple myeloma. Manag Care Inter Face. 2004;17:52–61.
- Hornberger J, Rickert J, Dhawan R, Liwing J, Aschan J, Löthgren M. The cost-effectiveness of bortezomib in relapsed/refractory multiple myeloma: Swedish perspective. Eur J Haematol. 2010;85:484–91. doi: 10.1111/j.1600-0609.2010.01526.x
- Möller J, Nicklasson L, Murthy A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: lenalidomide plus dexamethasone vs bortezomib. J Med Econ. 2011;14:690–7. doi: 10.3111/13696998.2011.611841
- The Publicity Medicine Prices of Jiangsu. Available from: http://yy.jspn.net (accessed October 2014).
- National Development and Reform Commission (NDRC). Available from: http://en.ndrc.gov.cn/ (accessed October 2014).
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20. doi: 10.1182/blood-2007-10-116129
- Ludwig H, Beksac M, Blade J, Cavenagh J, Cavo M, Delforge M, et al. Multiple myeloma treatment strategies with novel agents in 2011: a European perspective. Oncologist. 2011;16:388–403. doi: 10.1634/theoncologist.2010-0386
- van de Donk NW, Lokhorst HM, Dimopoulos M, Cavo M, Morgan G, Einsele H, et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37:266–83. doi: 10.1016/j.ctrv.2010.08.008
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology Multiple Myeloma (V2.2015). Available from: http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf (accessed September 2014).
- Multiple Myeloma Working Party of the Chinese Group. Guidelines for treatment of multiple myeloma. Zhonghua Nei Ke Za Zhi. 2008;47:869–72.
- Chinese Medical Association, Chinese Society of Hematology, Multiple Myeloma Working Party of the Chinese Group. Guidelines for treatment of multiple myeloma. Zhonghua Nei Ke Za Zhi. 2013;52:791–5.
- Peng LB, Tan CQ, Wan XM. Cost-utility analysis in China: differences and difficulties compared with developed countries. Pharmacoeconomics. 2007;25:619. doi: 10.2165/00019053-200725070-00007
- Ghatnekar O, Alvegard T, Conradi N, Lenhoff S, Mellqvist UH, Persson U, et al. Direct hospital resource utilization and costs of treating patients with multiple myeloma in Southwest Sweden: a 5-year retrospective analysis. Clin Ther. 2008;30:1704–13. doi: 10.1016/j.clinthera.2008.09.003
- Armoiry X, Fagnani F, Benboubker L, Facon T, Fermand JP, Hulin C, et al. Management of relapsed or refractory multiple myeloma in French hospitals and estimation of associated direct costs: a multi-centre retrospective cohort study. J Clin Pharm Ther. 2011;36:19–26. doi: 10.1111/j.1365-2710.2009.01153.x
- Koleva D, Cortelazzo S, Toldo C, Garattini L. Healthcare costs of multiple myeloma: an Italian study. Eur J Cancer Care (Engl). 2011;20:330–6. doi: 10.1111/j.1365-2354.2009.01153.x
- Gaultney JG, Franken MG, Tan SS, Redekop WK, Huijgens PC, Sonneveld P, et al. Real-world healthcare costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J Clin Pharm Ther. 2013;38:41–7. doi: 10.1111/jcpt.12020
- Teitelbaum A, Ba-Mancini A, Huang H, Henk HJ. Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data. Oncologist. 2013;18:37–45. doi: 10.1634/theoncologist.2012-0113
- Franken MG, Gaultney JG, Blommestein HM, Huijgens PC, Sonneveld P, Redekop WK, et al. Policymaker, please consider your needs carefully: does outcomes research in relapsed or refractory multiple myeloma reduce policymaker uncertainty regarding value for money of bortezomib? Value Health. 2014;17:245–3. doi: 10.1016/j.jval.2013.12.009