Abstract
Objective: Angiogenesis have implications in leukemia biology. Angiopoietin 1 (Ang 1) is an angiogenic cytokine which is essential in survival and proliferation of endothelial cells. Angiopoietin 2 (Ang 2) promotes dissociation of pericytes and increases vascular permeability and stromal derived factor 1 alpha (SDF 1α) which is a key player in stem cell traffic in the bone marrow (BM), has stimulating effects on angiogenesis as well. Here, we investigated the role of the leukemic BM microenvironment and specifically, the role of SDF 1α-CXCR4 and Ang 1/Ang 2–Tie 2 axes.
Methods: Here, Ang 1, Ang 2, and SDF 1α levels were measured in the BM plasma and in supernatants of mesenchymal stem/stromal cells (MSCs) of patients with ALL and compared with those of healthy controls.
Results: The results showed that at diagnosis, BM plasma levels of Ang 1 and SDF 1α were significantly low and Ang 2 was high when compared to control values. Remission induction was associated with an increase in Ang 1/Ang 2 ratio and SDF levels in BM plasma.
Discussion: The results suggest that BM microenvironment and leukemic cell–stroma interaction influences the secretion of Ang 1, 2 and SDF 1α, thus, may affect both angiogenesis, homing and mobilization of leukemic blasts.
Introduction
Acute lymphoblastic leukemia (ALL) is a malignant disorder of lymphoid progenitor cells with a peak prevalence in preschool ages.Citation1 Recent studies revealed that modifications within the bone marrow (BM) microenvironment play a major role in development and progression of leukemia.Citation2 The BM is a unique dynamic network of growth factors, cytokines, progenitor cells, stem cells, and stromal cells, providing a permissive environment for leukemogenesis and progression.Citation2 Crosstalk between leukemic cells and BM stroma including mesenchymal stem/stromal cells (MSCs) is critical in determining the outcome of leukemia. As well as maintaining physiological hematopoiesis, MSCs also have a role in providing suitable microenvironment for the proliferation of tumoral cells. In the last decade, there is an emerging concept that MSCs are one of the key players in chemotherapy resistance.Citation3
Angiopoietin (Ang)–Tie system is a predominant regulator of vascular integrity. Angs are ligands of the Tie 2 receptor. Ang 1 acts as an agonist and activates the Tie 2 signaling pathways, and Ang 2 acts as an antagonist specifically blocking the Ang 1 dependent activation of the same pathways.Citation4 Current data about contribution of Ang–Tie system to tumor angiogenesis are generally obtained from solid tumors.Citation5 Ang 1 which is expressed on perivascular cells like pericytes, vascular smooth muscle cells, fibroblasts, osteoblasts, and tumoral cellsCitation6–Citation8 is essential for the survival and proliferation of endothelial cells as well as for the formation and stabilization of new vessels.Citation5 Ang 1–Tie 2 interaction keeps vascular endothelial cells and hematopoietic stem cells (HSCs) in the quiescence phase.Citation5,Citation9 It is supposed that in a similar way Ang 1 is important in keeping leukemic stem cells in osteoblastic niche where they remain in quiescence phase.Citation10 Ang 2 which is expressed on endothelial cellsCitation11,Citation12 takes place when endothelium is activated and it functions as an Ang 1 antagonist promoting the dissociation of pericytes from pre-existing vessels and increase in vascular permeability.Citation5 Expression of Angs is upregulated in several tumors. Recent studies have shown that the ratio of Ang 1/Ang 2 has a critical role in the outcome of solid tumors and a decrease in this ratio in favor of Ang 2 is a marker of poor prognosis in many cancer types.Citation4
Stromal Derived Growth Factor 1α (SDF 1α/CXCL12) is mainly expressed by MSCs in various organs and tissues, such as the liver, lungs, lymphatic tissues, and BM.Citation13 SDF 1α-CXCR4 (receptor for SDF 1) signaling pathway is well known for its roles in homing of HSCs into BMCitation14 and regulation of primary tumor growth and metastasis.Citation15 SDF 1α also regulates the process of homing and engraftment of leukemic stem cells into the BM as well as it acts in leukemic cell survival, proliferation, differentiation. Inhibition of SDF 1α-CXCR4 leads to the differentiation and mobilization of leukemic blasts making them more sensitive to chemotherapy.Citation15 SDF 1α which plays a major role in governing stem cell traffic and all biological functions related to stem cell in BM microenvironment, also has important roles in angiogenesis. SDF 1α promotes angiogenesis in different ways such as functioning in homing and proliferation of endothelial progenitor cells in the tumoral region,Citation16,Citation17 inducing the secretion of matrix metalloproteinases which degrades extracellular matrix and activates the growth factors like basic fibroblast growth factor (bFGF)Citation18 and Interleukin 8 which is also an angiogenic factor.Citation19
Both BM stroma and leukemic blasts promote angiogenesis in ALL.Citation2 Up to date, contribution of angiogenic factors such as vascular endothelial growth factor and bFGF to leukomogenesis have been investigated in several trials.Citation20–Citation22 However the effect of Angs on pediatric ALL has not been studied and there are very limited data concerning stromal derived factor 1 alpha (SDF 1α) in ALL yet.Citation15,Citation23 Here, we investigated the role of the leukemic BM microenvironment and specifically, the role of SDF 1α-CXCR4 and Ang 1/Ang 2–Tie 2 axes. In this study, the levels of multiple angiogenic factors such as Ang 1, Ang 2, and SDF 1α were measured in BM plasma and supernatants of MSCs representing BM microenvironment and compared with healthy controls. Considering the favorable role of early response to treatment in ALL the changes in the levels of angiogenic cytokines and Ang 1/Ang 2 ratio before and after the remission induction therapy were assessed. In order to elucidate the contribution of MSCs to the release of the cytokines, MSCs supernatants were also tested for those parameters.
Patients and methods
Samples
In this prospective controlled trial, 20 newly diagnosed pediatric patients with ALL were included. Fifty one patients attended to Ankara Children Hematology Oncology Hospital between April 2011 and February 2013 and 20 of them whose marrow sample was available for the study were selected. Patients with ALL who had additional diseases affecting BM (Fanconi Aplastic anemia), relapsed ALL or previous stem cell transplantation were excluded. Eight age and sex matched healthy BM donors were selected as control group. After receiving Institutional Ethical Board approval, written informed consent was obtained from all participants’ parents/guardigans prior to their inclusion in the study.
The patients were diagnosed, classified, and treated according to the ALL-BFM 2000 protocol.Citation24–Citation26 BM samples were collected at the diagnosis and day +33 of the remission induction therapy. Blast percentages were determined microscopically using May-Grünwald Giemsa staining.
Plasma of the BM samples were isolated by centrifugation at 2500 rpm for 15 minutes and then stored at −80°C.
MSCs harvest and culture
Leukemic cells or mononuclear cells from controls were isolated by Biocoll seperating solution (density: 1.077 g/ml) (Biochrom, Berlin, Germany) density gradient centrifugation as previously described.Citation27 In order to expand MSCs, BM mononuclear cells were cultured in Dulbeco's Modified Eagle Medium (DMEM) supplemented with l-glutamin and 10% fetal bovine serum (FBS). The cultures were maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2 and medium was changed every 3 days. Adherent cells were harvested by Tripsin–EDTA enzyme at 70–80% confluency (passage 0) (Fig. A). This procedure was repeated until passage 2 (P2). In our cell culture laboratory, we mainly seeded 300 000 number of MSCs at the beginning of P2 and we mainly harvested approximately 600 000 MSCs at the end of the culture period. One milliliter supernatant of P2 culture medium was collected when the cells were at 70–80% confluency and then frozen at −80°C after filtering through 0.20 μm filter until enzyme-linked immunosorbent assay (ELISA) tests were performed.
Figure 1 (A) MSCs (P2) derived from bone marrow of acute lymphoblastic leukemia. (B) Adipogenic differentiation of MSCs (P2) derived from bone marrow of acute lymphoblastic leukemia (oil red O staining) (MSCc and adipogenic differentiation are shown in 10× magnification).
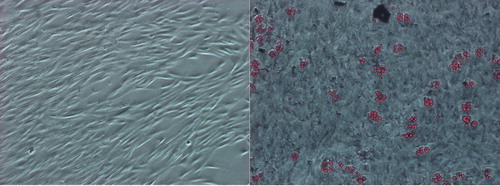
BM MSCs were characterized by flow cytometry (Becton Dickinson FACS Aria) using a specific CD antibody panel (Becton Dickinson and BioLegend) including hematopoietic (CD14, CD34, and CD45) and mesenchymal markers (CD73, CD105, and CD44) (data not shown). The MSCs were further characterized by their capacity to differentiate into adipogenic (Fig. B) and osteogenic lineages (data not shown) as previously described.Citation28 Briefly, adipogenic differentiation was induced in the expanded mesenchymal cell cultures by treatment with 1-methyl-3-isobutylxanthine, dexamethasone, insulin, and indomethacin. Induction was apparent by the accumulation of lipid-rich vacuoles within cells after 21 days that stain with Oil red O. The osteogenic differentiation was promoted in the expanded mesenchymal cell cultures by treatment with dexamethasone, β-glycerol phosphate, and ascorbate in the presence of 10% (v/v) FBS, which caused aggregate or nodule formation. After 21 days, cells stained with Alizarin Red.
ELISA
Cytokines levels in BM plasma and MSCs supernatants were measured by ELISA test which was performed using commercially available kits from Raybiotech systems according to the manufacturer's instructions. The cytokine levels and Ang 1/Ang 2 ratios were measured at diagnosis and after the remission induction therapy of patients and also measured in control group. Also, the cytokine level of conditioning medium which included FBS but no cell was measured. The minimum detectable dose stated by the manufacturer was 30 pg/ml for Ang 1, 10 pg/ml for Ang 2 and 80 pg/ml for SDF 1α.
Statistical analysis
All patients (T and B cell ALL) were considered in one group. The cytokine levels and Ang 1/Ang 2 ratios of the patients at diagnosis and after the remission induction therapy were compared with control group statistically. Mean ± SD and/or median (range) values were recorded for numeric variables. Number percent was used for categorical variables. Comparison of two independent groups was tested by Mann–Whitney in numerical variables and chi-square in categorical variables. Comparison of variables before and after treatment was tested by Wilcoxon. Kruskal–Wallis analysis was performed in order to compare the numerical variables according to the risk groups. Spearman rho correlation coefficient was used in order to investigate the relation between the numerical variables. The differences were statistically significant when P value was smaller than 0.05. IBM SPSS Statistics version 21.0 was used for the analysis.
Results
Mean age of patients and control groups was 7.4 ± 5.8 and 7.5 ± 4.0 years, respectively. Female/male ratio was 6/14 and 2/6 in patients and control group, respectively. The vast majority of patients were of B lymphocytic lineage. Seven patients were in low risk group, eighth in intermediate and five in high risk group. High risk group existed exclusively of pre B cell ALL patients whereas one patient with T lymphocytic lineage was in high risk group (Table ). All patients except two achieved remission after remission induction therapy.
Table 1 Demographic data of patients and controls are demontrated. (A) The age and gender of the patients and controls are stated. (B) Differential blood count, BM blast percentage at diagnosis, ALL subtypes, chromosome abnormalities, risk groups and remission status (after induction therapy) of patients are stated. Two patients had t(11q23) which was unfavorable and two had t(12;21) which was favorable
At diagnosis (before treatment) BM plasma levels of Ang 1 and SDF 1α were significantly low (P < 0.001 and P = 0.023, respectively) and Ang 2 was high (P < 0.001) when compared to control values (Table ). Following the remission induction therapy at day +33 levels of Ang 1 and SDF1α increased (P = 0.002 and P = 0.006, respectively) while level of Ang 2 decreased (P = 0.030) when compared to the levels at diagnosis. At the time of diagnosis BM plasma Ang 1/Ang 2 ratio was lower than the control group (P < 0.001) and at day +33 the ratio increased significantly when compared to the levels at diagnosis (P = 0.001).
Table 2 Cytokine levels of patients at time of diagnosis and after induction therapy (on day +33) and control groups. The cytokine levels were measured both in BM plasma and MSCs supernatant
A positive correlation between Ang 2 levels in BM plasma and supernatants was detected (r = 0.520, P = 0.010). Neither Ang 1 nor SDF 1α levels were correlated between BM plasma and MSC supernatants.
The pattern of Ang 1 and Ang 2 secretion from P2 MSCs was not different among patients and controls (Table ). However Ang 1/Ang 2 ratio was significantly lower in supernatants at diagnosis (P = 0.035) when compared to control levels, SDF 1α levels did not show a statistically significant change in supernatant samples.
While all patients except two achieved remission after remission induction therapy, statistical analysis to compare the changes in the levels of the angiogenic cytokines with remission status was not available.
Any correlation between the levels of cytokines and the patients’ leukocyte, blast count, hemoglobin level and platelet counts and the blast percentage of the BM were not detected at diagnosis and on day +33.
Discussion
Setting up the experimental conditions mimicking human BM microenvironment is a challenging issue due to the complex dynamic cell–cell–matrix–soluble factor interactions and the consistently changing physical conditions in vitro. Partial data concerning microenvironment are obtained from co-culture studies. Examination of BM plasma is a simple measure in projecting the in vivo BM microenvironment. In present study, some angiopoietic cytokines in BM of patients with ALL were tested in order to assess the role of these cytokines and MSC supernatants were tested in order to understand the contribution of MSCs to the release of these cytokines. It was demonstrated that at diagnosis the levels of Ang 1 and SDF 1α were low while Ang 2 level was high in the BM microenvironment represented by BM plasma levels. After the remission induction phase Ang 1 and SDF 1α levels increased and Ang 2 were found to be decreased. However those soluble factors in MSCs supernatants did not reveal a statistically significant change from control samples. Of interest, a low Ang 1/Ang 2 ratio was also present in MSCs supernatants obtained from BM samples at diagnosis. In order to obtain sufficient number of cells for in vitro studies, MSCs were expanded in culture for several weeks. Therefore, the data obtained from MSCs do not picture in vivo conditions exactly due to the absence of interacting cells and factors in in vitro conditions. Additionally due to the use of fetal calf serum for culture expansion the secretory profile does not represent in vivo levels but give information about the secretory capacity of the cells which were exposed to in vivo leukemic environment when compared with cells obtained from healthy donors. Here we investigated the functional/secretory and dynamic features of MSCs and showed that they do not differ between the time of diagnosis and after induction therapy. Conforti et al. studied the morphological features of MSCs derived from pediatric patients with ALL and demonstrated that MSCs at diagnosis do not differ from those obtained during treatment.Citation29 However, Mallampati et al.Citation30 who studied a mouse BCR-ABL+ ALL model observed that tyrosine kinase inhibitors induce MSC-mediated resistance and MSCs may play an essential role in activation of an alternative survival signaling pathway in leukemic cells that protects leukemic cells from chemotherapy.
The effects of Ang 1 and Ang 2 on tumor angiogenesis have been studied in acute myeloid leukemia (AML),Citation31–Citation33 chronic lymphocytic leukemia,Citation34 multiple myeloma,Citation35 and myelodysplastic syndrome (MDS).Citation36 To our knowledge this is the first study about the effect of angiopoietins in ALL.
Studies with adult patients with AML usually revealed that Ang 2 is a prognostic factor and high levels of Ang 2 is related with poor outcome.Citation32,Citation37,Citation38 Likewise Hatfield et al.Citation33 demonstrated that serum Ang 2 levels of 7 untreated adult patients with ALL were higher than control. However Cheng et al.Citation36 studied the levels of Ang 1, Ang 2, and Tie 2 levels of 208 adult patients with MDS in BM and found that the level of Ang 2 was low whereas Ang 1 and Tie 2 was high. They also confirmed that acute leukemic transformation was more frequent and prognosis was poorer in patients with high Ang 1 levels.
In the present study, Ang 1/Ang 2 ratio of BM plasma was low at the time of diagnosis and increased after the remission induction therapy. Interaction of Ang 1 and Ang 2 and Ang 1/Ang 2 ratio have a critical role in vascular homeostasis in BM stem cell traffic.Citation39,Citation40 Furthermore, it is established that Ang 1/Ang 2 ratio is a prognostic factor in solid tumors.Citation4 However it has not been demonstrated in acute leukemias. Since the number of poor responder subjects was relatively small and the follow-up period was short the correlation of Ang 1/Ang 2 ratio with prognosis could not be evaluated. Hypothetically, the low ratio of Ang 1/Ang 2 at diagnosis is related to leukemic transformation, and increase in this ratio after induction therapy may be attributed to the response to the therapy. Also, hypothetically, the interaction between the leukemic blasts and MSCs might influence Ang 1/Ang 2 ratio. Further studies including co-culture studies are needed to determine the role of Ang 1/Ang 2 ratio in leukemic BM microenvironment. We suggest that the low ratio of Ang 1/Ang 2 at beginning and increase of it after the induction therapy, to some extent might be the nature of pediatric ALL.
Physiologically there is a balance between angiogenic and anti-angiogenic factors. Angiogenic switch defines the disequilibrium resulting in increased/decreased angiogenesisCitation41 depending on the dominant side. Hypothetically, the prepotency of Ang 2 at the time of diagnosis leads to an increase in angiogenesis, vascular permeability and peripheric mobilization of the blasts. We suggest that after remission induction therapy with reduction of blastic cells Ang 1 dominates resulting in stabilization of the newly formed vessels, decrease in vascular permeability and homing of HSCs and residual leukemic stem cells into osteoblastic niche where they remain in quiescent phase. Szmigielska-Kaplon et al.Citation42 have studied Angs in hematopoietic stem cell mobilization in patients with hematological malignancies and found out that at the time of mobilization levels of Ang 1 was low and Ang 2 was high, when compared with basal levels. Dynamic ratio of Ang 1/Ang 2 might have a role in stem cell (MSCs, endothelial stem cells and HSCs) traffic in BM microenvironment affecting the behavior of leukemic stem cell and might have important roles about angiogenesis, chemotherapy resistance and prognosis in leukemia. Further studies are needed to evaluate these hypotheses.
In literature, SDF 1α has been studied mostly in solid tumors which have more stromal tissue than BM and levels of it were found to be higher than controls.Citation43,Citation44 There are a few studies concerning SDF 1α in ALL. Mowafi et al.Citation45 and Ge et al.Citation46 showed in the pediatric and adolescent/young adults respectively that serum SDF 1α levels were higher in pediatric patients with ALL at diagnosis and similar to control after therapy. In our study, we measured levels of SDF 1α in BM plasma and demonstrated that patients had lower levels at diagnosis and higher levels after induction therapy, when compared to controls. Van den Bek et al. also demonstrated that CXCL12 levels of 11 pre B cell ALL patients in BM were lower than controls at diagnosis. It is well established that SDF 1α is critical in homing of progenitor and stem cells into BM niches.Citation47 Lymphoid progenitor cells express high levels of CXCR4 on surface which makes them a suitable target for SDF 1α. Activation of SDF 1α/CXCR4 leads to adhesion, chemotaxis, proliferation, and survival of leukemic stem cells.Citation15 Inhibition of this pathway leads to the differentiation and mobilization which makes leukemic cells more sensitive to treatment.Citation15
In conclusion, in pediatric ALL at the time of diagnosis high levels of Ang 2 and low levels of Ang 1 and SDF 1α all together may have an effect on opening up the BM niches and promoting the proliferation and peripheral mobilization of blastic population. After the induction therapy low levels of Ang 2 and high levels of Ang 1 and SDF 1α may close down the niches and ends up with homing of leukemic blasts into the niche where they remain in quiescent phase. These results point out that factors related to angiogenesis have important roles in governing stem cell traffic by means of vessel stability. Ang 1, Ang 2, and SDF 1α may have an effect not only on angiogenesis but also homing of leukemic blasts into BM and peripheral mobilization. Taken together, alteration of Ang 1, Ang 2, and SDF 1α expression could be potentially developed as biomarkers for monitoring the effectiveness of chemotherapy. Future experimental studies in co-culture conditions may reveal the direct effect of MSCs to the secretion of angiogenic factors which may have implications in development of targeted therapies.
Disclaimer statements
Contributors All of authors have contributed to the paper.
Funding This work was supported by Turkish Society of Hematology (Grant No. 12/344)
Conflict of interest There was no conflict of interest in any of the authors who were involved in this study.
Ethics approval We received Institutional Ethical Board approval from Ankara University.
Acknowledgements
The study was funded by Turkish Hematology Association. We would like to express our thanks to the staff members of stem cell laboratory in Ankara Pediatric Hematology Oncology Hospital and in Hacettepe University School of Medicine (PediStem) who contributed to the scientific review and to department of HSCT for their contribution to obtain stem cell donors. We also thank Prof. Dr Emin Kansu for his valuable discussion in preparation of this manuscript.
References
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–78. doi: 10.1056/NEJMra052603
- Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23(12):2233–41. doi: 10.1038/leu.2009.175
- Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest. 2007;117(4):1049–57. doi: 10.1172/JCI30235
- Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204(1):1–10. doi: 10.1002/path.1618
- Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18–26. doi: 10.1016/j.canlet.2012.08.018
- Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biochem. 2001;276(37):34990–8.
- Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Cir Res. 2000;86(1):24–9. doi: 10.1161/01.RES.86.1.24
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–9. doi: 10.1016/S0092-8674(00)81812-7
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004
- Ichihara E, Kaneda K, Saito Y, Yamakawa N, Morishita K. Angiopoietin1 contributes to the maintenance of cell quiescence in EVI1(high) leukemia cells. Biochem Bio Res Co. 2011;416(3–4):239–45. doi: 10.1016/j.bbrc.2011.10.061
- Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biochem. 1999;274(22):15732–9.
- Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Cir Res. 1998;83(8):852–9. doi: 10.1161/01.RES.83.8.852
- Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23(1):43–52. doi: 10.1038/leu.2008.299
- Broxmeyer HE, Cooper S, Kohli L, Hangoc G, Lee Y, Mantel C, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170(1):421–9. doi: 10.4049/jimmunol.170.1.421
- Tavor S, Petit I. Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia? Semin Cancer Biol. 2010;20(3):178–85. doi: 10.1016/j.semcancer.2010.07.001
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48. doi: 10.1016/j.cell.2005.02.034
- Hoh BL, Hosaka K, Downes DP, Nowicki KW, Wilmer EN, Velat GJ, et al. Stromal cell-derived factor-1 promoted angiogenesis and inflammatory cell infiltration in aneurysm walls. J Neurosurg. 2014;120(1):73–86. doi: 10.3171/2013.9.JNS122074
- Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol. 2000;28(11):1274–85. doi: 10.1016/S0301-472X(00)00532-4
- Li KC, Huang YH, Ho CY, Chu CY, Cha ST, Tsai HH, et al. The role of IL-8 in the SDF-1alpha/CXCR4-induced angiogenesis of laryngeal and hypopharyngeal squamous cell carcinoma. Oral Oncol. 2012;48(6):507–15. doi: 10.1016/j.oraloncology.2012.01.006
- Yetgin S, Yenicesu I, Cetin M, Tuncer M. Clinical importance of serum vascular endothelial and basic fibroblast growth factors in children with acute lymphoblastic leukemia. Leukemia Lymphoma. 2001;42(1–2):83–8. doi: 10.3109/10428190109097679
- Schneider P, Vasse M, Legrand E, Callat MP, Vannier JP. Have urinary levels of the angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, a prognostic value in childhood acute lymphoblastic leukaemia? Br J Haematol. 2003;122(1):163–4. doi: 10.1046/j.1365-2141.2003.04395_4.x
- Lyu CJ, Rha SY, Won SC. Clinical role of bone marrow angiogenesis in childhood acute lymphocytic leukemia. Yonsei Med J. 2007;48(2):171–5. doi: 10.3349/ymj.2007.48.2.171
- van den Berk LC, van der Veer A, Willemse ME, Theeuwes MJ, Luijendijk MW, Tong WH, et al. Disturbed CXCR4/CXCL12 axis in paediatric precursor B-cell acute lymphoblastic leukaemia. Br J Haematol. 2014;166(2):240–9. doi: 10.1111/bjh.12883
- Flohr T, Schrauder A, Cazzaniga G, Panzer-Grumayer R, van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22(4):771–82. doi: 10.1038/leu.2008.5
- Stanulla M, Schaeffeler E, Flohr T, Cario G, Schrauder A, Zimmermann M, et al. Thiopurine methyltransferase (TPMT) genotype and early treatment response to mercaptopurine in childhood acute lymphoblastic leukemia. JAMA. 2005;293(12):1485–9. doi: 10.1001/jama.293.12.1485
- Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–14. doi: 10.1182/blood-2009-10-248146
- Sbaa-Ketata E, Vasse M, Lenormand B, Schneider P, Soria C, Vannier JP. Fibronectin increases the migration induced by stromal cell-derived factor-1 alpha (SDF-1 alpha) in pre-B acute lymphoblastic leukemia cells. Eur Cytok Net. 2001;12(2):223–30.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143
- Conforti A, Biagini S, Del Bufalo F, Sirleto P, Angioni A, Starc N et al. Biological, functional and genetic characterization of bone marrow-derived mesenchymal stromal cells from pediatric patients affected by acute lymphoblastic leukemia. PloS One. 2013;8(11):e76989. doi: 10.1371/journal.pone.0076989
- Mallampati S, Leng X, Ma H, Zeng J, Li J, Wang H, et al. Tyrosine kinase inhibitors induce mesenchymal stem cell-mediated resistance in BCR-ABL+ acute lymphoblastic leukemia. Blood. 2015;125(19):2968–73. doi: 10.1182/blood-2014-05-576421
- Loges S, Heil G, Bruweleit M, Schoder V, Butzal M, Fischer U, et al. Analysis of concerted expression of angiogenic growth factors in acute myeloid leukemia: expression of angiopoietin-2 represents an independent prognostic factor for overall survival. J Clin Oncol. 2005;23(6):1109–17. doi: 10.1200/JCO.2005.05.058
- Hou HA, Chou WC, Lin LI, Tang JL, Tseng MH, Huang CF, et al. Expression of angiopoietins and vascular endothelial growth factors and their clinical significance in acute myeloid leukemia. Leuk Res. 2008;32(6):904–12. doi: 10.1016/j.leukres.2007.08.010
- Hatfield KJ, Hovland R, Oyan AM, Kalland KH, Ryningen A, Gjertsen BT, et al. Release of angiopoietin-1 by primary human acute myelogenous leukemia cells is associated with mutations of nucleophosmin, increased by bone marrow stromal cells and possibly antagonized by high systemic angiopoietin-2 levels. Leukemia. 2008;22(2):287–93. doi: 10.1038/sj.leu.2404985
- Vrbacky F, Smolej L, Vroblova V, Pekova S, Hrudkova M, Cervinka M, et al. Angiopoietin-2 mRNA expression is increased in chronic lymphocytic leukemia patients with poor prognostic features. Hematology. 2010;15(4):210–4. doi: 10.1179/102453309X12583347113898
- Pappa CA, Tsirakis G, Samiotakis P, Tsigaridaki M, Alegakis A, Goulidaki N, et al. Serum levels of angiopoietin-2 are associated with the growth of multiple myeloma. Cancer Invest. 2013;31(6):385–9. doi: 10.3109/07357907.2013.800093
- Cheng CL, Hou HA, Jhuang JY, Lin CW, Chen CY, Tang JL, et al. High bone marrow angiopoietin-1 expression is an independent poor prognostic factor for survival in patients with myelodysplastic syndromes. Br J Cancer. 2011;105(7):975–82. doi: 10.1038/bjc.2011.340
- Schliemann C, Bieker R, Thoennissen N, Gerss J, Liersch R, Kessler T, et al. Circulating angiopoietin-2 is a strong prognostic factor in acute myeloid leukemia. Leukemia. 2007;21(9):1901–6. doi: 10.1038/sj.leu.2404820
- Orkin SH, Look AT, Lux SE, Ginsburg D, Nathan DG. Angiogenesis. In: LC J, (eds.) Oncology of infancy and childhood 1. 1st ed. Philadelphia: Saunders Elsevier; 2009. p. 27–40.
- Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118(Pt 4):771–80. doi: 10.1242/jcs.01653
- Thomas M, Felcht M, Kruse K, Kretschmer S, Deppermann C, Biesdorf A, et al. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J BioChem. 2010;285(31):23842–9.
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi: 10.1038/nrc1093
- Szmigielska-Kaplon A, Krawczynska A, Czemerska M, Pluta A, Cebula-Obrzut B, Szmigielska K, et al. Angiopoietins in haematopoietic stem cell mobilisation in patients with haematological malignancies. Blood Transfus. 2015;13(1):102–8.
- Terai S, Fushida S, Tsukada T, Kinoshita J, Oyama K, Okamoto K, et al. Bone marrow derived “fibrocytes” contribute to tumor proliferation and fibrosis in gastric cancer. Gastric Cancer. 2015;18(2):306–13. doi: 10.1007/s10120-014-0380-0
- Zhao S, Chang SL, Linderman JJ, Feng FY, Luker GD. A comprehensive analysis of CXCL12 isoforms in breast cancer. Translational Oncol. 2014 May 13. pii:S1936-5233(14)00021-7.
- Mowafi F, Cagigi A, Matskova L, Bjork O, Chiodi F, Nilsson A. Chemokine CXCL12 enhances proliferation in pre-B-ALL via STAT5 activation. Pediatr Blood Cancer. 2008;50(4):812–7. doi: 10.1002/pbc.21370
- Ge J, Hu Y, Gui Y, Hou R, Yang M, Zeng Q, et al. Chemotherapy-induced alteration of SDF-1/CXCR4 expression in bone marrow-derived mesenchymal stem cells from adolescents and young adults with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2014;36(8):617–23. doi: 10.1097/MPH.0000000000000220
- Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, et al. Rapid and efficient homing of human CD34(+)CD38(−/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2 m(null) mice. Blood. 2001;97(10):3283–91. doi: 10.1182/blood.V97.10.3283
