Abstract
Introduction: Thrombotic thrombocytopenic purpura (TTP) is characterized by a reduction in the von Willebrand cleavage protein ADAMTS-13, mainly as a consequence of autoimmunity. Plasma exchange (PEx) is standard, achieving complete remission (CR) in 77–83% of cases, but rates are variable depending on ADAMTS-13 activity and relapse is frequent in patients with <10%. Thus, an effective front-line immunosuppressive treatment is needed.
Materials and methods: We administered PEx daily until CR and rituximab 100 mg/dose/week for 4 consecutive weeks to 10 patients with a first TTP episode and 1 relapsed patient (8 females (72%) and 3 males (28%)). Median age was 34 years (15–46) and laboratory parameters at diagnosis were as follows: platelets 11 × 109/l (range 7–27.4 × 109/l), lactate dehydrogenase 1822 U/l (range 705–8220 U/l, normal 70–180 U/l), and haemoglobin 6 g/dl (range 4.2–11.8 g/dl). ADAMTS-13 activity was determined in eight patients and was <10% in all. ADAMTS-13 autoantibody titre was determined in seven patients and was >15 units/ml in all (ref: negative <12, undetermined 12–15, positive >15 units/ml); Shiga toxin was negative in all patients. The median number of PEx until CR was 7 (range 4–12); prednisone 1 mg/kg was administered to six patients.
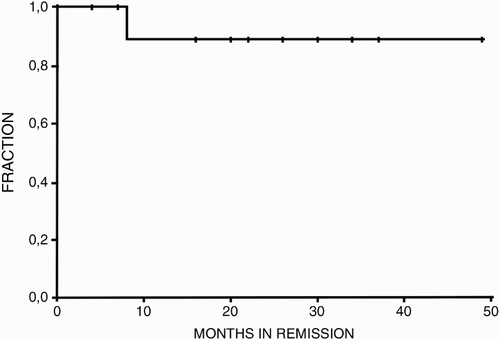
Results: The median follow-up was 22 months (range 4–49) and the estimated 2-year relapse-free survival was 89%; one HIV+ patient relapsed at 8 months follow-up. No complications related to PEx or rituximab were reported.
Conclusions: Our study suggests that low-dose rituximab and PEx are effective as front-line treatment for acute TTP; however, a prospective trial is needed to demonstrate whether low-dose rituximab is as effective as the conventional dose.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is characterized by a reduction in the von Willebrand cleavage protein ADAMTS-13, caused in the majority of cases by autoimmunity. It is an illness of rapid and fatal evolution if effective treatment is not rapidly established.Citation1 Before the introduction of plasma exchange (PEx), only 10% of patients survived, and since PEx proved to be superior to plasma infusion, it has become the standard treatment, achieving remission rates of between 77 and 83%. However, this figure varies considerably, depending on the clinical context and ADAMTS-13 activity.Citation2,Citation3 The number of PEx procedures required to achieve remission is also variable and it has been reported that patients with <10% ADAMTS-13 underwent a median of 19 PEx (range 2–79) to achieve remission.Citation4 Relapse is still frequent, mainly in patients with <10% ADAMTS-13 activity, with rates of between 34 and 41%. In the majority of these patients, the presence of an inhibitor against ADAMS-13 is detected and, therefore, an effective first-line immunosuppressive treatment is needed to effectively prevent relapses.Citation4
Rituximab (MabThera; Roche Pharmaceuticals) effectively depletes B lymphocytes and has been used in the treatment of autoimmune diseases and haematological disorders.Citation5,Citation6 Originally, it was used as a second-line therapy in patients with relapsed and refractory TTP, and was shown to be effective in achieving remission and reducing relapse rates.Citation7–Citation9 It has also been used as frontline treatment with similar results.Citation10–Citation12
Concerns have been raised about the fact that rituximab may actually be removed after plasmapheresis. In a study analysing the pharmacodynamics of rituximab in TTP patients, it was shown that although the drug is removed from the circulation, effective B cell depletion may be achieved after a brief exposure to a dose of 375 mg/m², and that the cumulative effect of subsequent doses contributes to this effect. The authors suggested that due to the rapid depletion of peripheral B cells after a single dose, this might be greater to what is required but concerns exists that by reducing the dose, depletion might not be as effective in lymph nodes, spleen, or bone marrow as with conventional doses.Citation13 In this respect, low-dose rituximab was found to be effective in TTP in our previous study of four patients treated with this protocolCitation10 and in other autoimmune diseases such as idiopathic thrombocytopenic purpura and autoimmune haemolytic anaemia.Citation14–Citation16
In this report, we document the long-term clinical evolution of our four previously reported patientsCitation10 and of seven additional cases with a first TTP episode treated with PEx and rituximab at 100 mg/week×4 as a first-line treatment.
Materials and methods
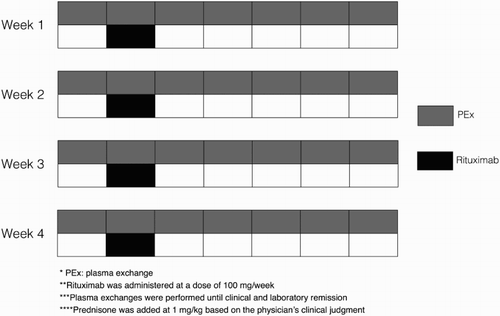
All patients >15 years diagnosed with a first TTP episode from March 2011 to March 2015 in our institution were included. Diagnosis was based on the presence of thrombocytopenia plus schistocytes in peripheral blood, and a negative Coombs test. All patients received daily PEx with 1–2 volume exchanges until clinical and laboratory remission (platelets >100 000 and normal lactate dehydrogenase (LDH) for 2 consecutive days) and rituximab at 100 mg/dose/week for 4 consecutive weeks, starting the first dose immediately after the second or third PEx (Fig. ). Some patients received prednisone at 1 mg/kg based on the physiciańs clinical judgment. ADAMTS-13 activity was measured by chromogenic assay TECHNOCLONE, TECHNOZYM ADAMTS-13 ACTIVITY®, Wien (Technoclone GmbH, Bruner Str 67, 1230 Wien, Austria), and was recorded as a percentage in comparison to that of normal pooled plasma. Antibodies against the protease were determined by solid-phase immunoanalysis with TECHNOZYM ADAMTS-13 INH® assay.
The clinical characteristics, number of PEx required to achieve complete remission (CR), relapses, and length of remission were recorded. Relapse was defined as readmission with thrombocytopenia (<100 × 109/l), schistocytes in the peripheral blood, and ADAMTS-13 activity <10%, with or without new symptoms after discharge from an acute episode. All patients were followed weekly for the first 4 weeks after hospital discharge, then monthly. We separated our cohort into two groups and report their outcome independently; the first group included our four originally treated patients and their long-term outcome. The second group included seven patients with a first TTP episode. We collected all relevant data into a single table and report the laboratory results and clinical characteristics as one cohort. Written consent was obtained from all patients or their guardians when unable to consent. A descriptive analysis was performed, obtaining medians and ranges. Relapse-free survival was determined using the Kaplan–Meier method.
Results
Patient characteristics
In the first group, three patients with a first TTP episode and another with relapsing disease were treated as described previously (Patients 1–4 in Table ). Three patients have since been in CR for a median of 37 months (range 34–49) and one diagnosed with HIV was lost to follow-up at month 30 of CR. Of note, all patients have since had normal complete blood counts without neurologic, renal, or any other sequelae related to the TTP episode.
Table 1 Clinical characteristics and laboratory findings
The second group (patients 5–11 in Table ) comprised seven patients with a first TTP episode (three men (43%) and four women (57%)). The median age was 35 years (range 21–46). The median follow-up for this group was 16 months (range: 4–26) and all patients had severe ADAMTS-13 deficiency (0% activity in all of them).
Considering all patients as one cohort, the median follow-up was 22 months (range 4–49) and for patients evaluable for ADAMTS-13 deficiency (n = 8), it was 18 months (range 4–34).
With respect to the clinical presentation of all patients, five presented with numbness and mucocutaneous bleeding, and two with petechiae and gingival bleeding with no neurological manifestations. Only two patients had renal failure at diagnosis (creatinine > 1.2), none presented with fever or cardiac involvement. Four patients had additional comorbidities (two had diabetes mellitus and one concomitant hypertension, other had hypothyroidism, and one was HIV+).
Laboratory parameters
The median laboratory parameters at diagnosis of the 11 patients were: platelet count 11 × 109/l (range 7–27.4 × 109/l, normal value 150–400 × 109/l), LDH 1822 U/l (range 705–8220 U/l, normal range 70–180 U/l), and haemoglobin 6.5 g/dl (range 4.2–11.8 g/dl, normal range 12–14 g/l). ADAMTS-13 activity was determined in eight patients at diagnosis and it was <10% (normal range 40–130%) in all cases. The ADAMTS-13 autoantibody title was determined in seven patients, being >15 units/ml (median 46, range 21.3–124.8, reference range: negative <12, undetermined 12–15, positive > 15 units/ml) in every case. Shiga toxin was negative in all patients.
Management
All 11 patients received rituximab 100 mg/dose/week for 4 consecutive weeks as per the protocol, starting the first dose immediately after the second or third PEx (Fig. ). The median number of plasmapheresis exchanges until remission was 7 (range 4–12) and prednisone at 1 mg/kg was administered in six cases.
Relapse-free survival, relapse, and adverse effects
The estimated 2-year relapse-free survival for the whole group was 89% (Fig. ). One HIV+ male patient suffered a relapse at 8 months of follow-up. A high antibody titre against ADAMTS-13 was detected at relapse with no ADAMTS-13 activity and the patient was retreated with PEx (three procedures) and low-dose rituximab 100 mg/dose/week for 4 consecutive weeks, achieving clinical remission. The patient has currently been in clinical remission for 2 months. There were no complications related to plasmapheresis or rituximab infusion.
Discussion
Owing to the favourable long-term outcome of our four TTP patients previously treated and reported with this protocol, we continued to explore its utility and validated our previous results by treating all subsequent patients diagnosed with TTP at our institution with the same protocol. Currently, the use of rituximab as front-line therapy in TTP is off-label, but due to favourable results achieved in patients with an inhibitor against ADAMTS-13, this premise should be reconsidered. There have been several reports using rituximab as front-line therapy at conventional doses. In a study that sought to identify factors associated with response to treatment, it was found that early administration of rituximab (<3 days) from diagnosis was associated with faster clinical remission, fewer PEx procedures, and shorter hospital stay; furthermore, rituximab given as prophylaxis after attaining clinical response prevented relapses.Citation12
There has been considerable debate regarding the optimal dose of rituximab for treating autoimmune diseases, and, although there is not a study that has proven that the 100 mg/m2 dose is as effective as the 375 mg/m2 dose in depleting B-cell population, there are reports of effective depletion and durable clinical responses utilizing this treatment protocol. In 2008, Zaja et al. evaluated the clinical response to rituximab 100 mg weekly in four doses in 28 adults with immune thrombocytopenic purpura (ITP). The assumption that the B cell population may be smaller in patients with autoimmune diseases than in neoplasia supported this idea and, in this study, effective B cell depletion was achieved with this dose.Citation17 We extrapolated this result to a clinical trial evaluating the efficacy of the same dose of rituximab in association with steroids in ITP patients, finding similar results.Citation18 Furthermore, we have also tested low-dose rituximab in combination with alemtuzumab in patients with steroid refractory ITP and haemolytic anaemia, achieving satisfactory responses.Citation19 All the aforementioned studies support the premise that low-dose rituximab might be safe and effective in other autoimmune diseases, such as TTP. It is important to highlight that, in our study, the median of plasmapheresis sessions needed to achieve clinical remission (7, range 4–12) was lower than that reported for patients with <10% ADAMTS-13 activity (19, range 2–79).Citation4 This result supports the hypothesis that depleting the peripheral B cell population aids in the process of clearing the inhibitor, and, as standard treatment dictates, supplementation of fresh plasma with active ADAMTS-13 by PEx leads to faster clinical remission. Six of our patients received steroids as a concomitant treatment based on the clinician's judgment. However, it is unlikely that this factor contributed greatly to their clinical improvement because steroids were included in the treatment of TTP in patients with severe ADAMTS-13 deficiency in other studies, and response rates were lower with more PEx sessions needed to achieve CR compared with that reported here.Citation4 Considering relapses in a study that included 60 patients with severe ADAMTS-13 deficiency, 47 (78%) of them survived but 16 relapsed. Ten initial relapses (63%) occurred within the first year and as many as 88% within 4 years.Citation4 The median follow-up for patients with severe ADAMTS-13 deficiency in our series was 18 months (range 4–34), and only one HIV+ patient relapsed after 8 months; accordingly, patients with HIV and TTP should be treated the same way since responses to PEx are the same in both groups.Citation20,Citation21
To the best of our knowledge, there have been no randomized controlled trials with high-quality evidence analysing the use of rituximab as first-line therapy. However, Scully et al. reported their results of a study comparing 40 patients who received rituximab as front-line therapy with 40 historical controls treated conventionally. Controls were matched for sex, ethnicity, and number of relapses. The CR rate was 93% (37/40) in patients initially treated with rituximab and 95% (38/40) in historical controls; relapse rates were 11 and 55%, respectively. No relapses occurred during 12 months of follow-up in the rituximab group and the median time to relapse was 27 months. In the historical controls, the median time to relapse was 18 months (range 3–60 months) after admission and there were fewer hospitalization days in the rituximab-treated group.Citation7
Interestingly, the patient who relapsed in our study group was HIV+ and was not taking antiretrovirals at discharge from the first TTP episode. At relapse, a high autoantibody titre against ADAMTS-13 was detected. We started the same treatment protocol and the patient achieved rapid clinical remission once PEx and low-dose rituximab were initiated, and we are currently evaluating the capacity of antiretrovirals to prevent relapse after 4 months of follow-up.
We recognize the limitations of our study as it was retrospective in nature, the number of patients included was small, and the frequency of corticosteroid use was not controlled. It is important to highlight that the clinical characteristics of our patients at presentation resemble TTP cases of a mild phenotype as no serious renal cardiac or neurological features were seen; also they had a rapid response to PEx. Nonetheless, severe ADAMTS-13 activity depletion was seen in all evaluable patients with a high autoantibody titre; this observation suggest that clinical presentation might not be directly related to ADAMTS-13 activity.
Given the relapse rates previously described in patients with severe ADAMTS-13 deficiency and that B cell depletion after rituximab use is effective 9–18 months post-treatment,Citation7 our median follow-up appears to be adequate for the evaluation of relapses or exacerbations. Of note, clinical remission in 10 of our patients (one lost to follow-up at month 30) has been sustained and prolonged.
Subsequent determinations of ADAMTS-13 levels were not performed due to the fact that is not part of our institutional protocol to make new determinations once the patient has achieved clinical remission. The value of measuring ADAMTS-13 activity during remission aside from clinical research is uncertain; in the study by Kremer-Hovinga et al.Citation4 of 41 patients with severe ADAMTS-13 deficiency, 8 had ADAMTS-13 activity ranging from 5 to 15% and were in clinical remission.Citation4 Nonetheless, ADAMTS-13 measurements in follow-up are made for clinical research and in patients with suspected relapse with thrombocytopenia, anaemia, or clinical symptoms, also, we acknowledge that some clinicians might consider to treat with pre-emptive rituximab therapy an asymptomatic patient with normal blood counts and ADAMTS-13 activity <10%.Citation22 On the other hand, considering a prospective study with low-dose rituximab and its effect on ADAMTS-13 activity would require serial ADAMTS-13 determinations.
At the present time, PEx remains standard for patients with TTP but effective methods to prevent relapses are needed. Although rituximab has not been approved for the treatment of TTP, its off-label use with increasing evidence, showing efficacy for initial treatment of acute or refractory episodes, and even as prophylaxis in asymptomatic patients with severe ADAMTS-13 deficiency, should support reconsidering its indication. However, we acknowledge that the appropriate role of rituximab in the management of TTP remains uncertain.Citation23
Our study suggests that front-line low-dose rituximab is promising for the treatment of TTP by reducing relapse rates and PEx procedures to achieve clinical remission.
Disclaimer statements
Contributors A.V.M. wrote the manuscript and reviewed final draft. M.P.L. wrote manuscript and obtained information. O.G.C.R. reviewed clinical data and review final draft. L.V.M. wrote manuscript. J.C.J.P. wrote manuscript and reviewed clinical data. A.G.D. wrote manuscript, obtained information. F.D.S. reviewed final draft, organized table and graphic. O.G.L.L. wrote manuscript, obtained clinical information. P.C.P. obtained clinical data and follow up. G.S.D. obtained clinical data. D.G.A. designed the study, wrote manuscript and approved final draft.
Funding Departamento de Hematología del Hospital Universitario “Dr. José Eleuterio González”.
Conflict of interest None of the authors have any conflict-of-interest to declare.
Ethics approval Because of the nature of the study (retrospective), there was no need for ethics approval.
References
- George JN. How I treat patients with thrombotic thrombocytopenic purpura. Blood. 2010;116(20):4060–9. doi: 10.1182/blood-2010-07-271445
- Amorosi E, Ultmann J. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966;45(2):139–59. doi: 10.1097/00005792-196603000-00003
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med. 1991;325(6):393–7. doi: 10.1056/NEJM199108083250604
- Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500–11; quiz 662. doi: 10.1182/blood-2009-09-243790
- Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237(1):264–83. doi: 10.1111/j.1600-065X.2010.00945.x
- Provan D, Newland AC, Amadori S, Stasi R. Rituximab in the treatment of autoimmune haematological disorders. Br J Haematol. 2008;143(2):294; author reply 5. doi: 10.1111/j.1365-2141.2008.07326.x
- Scully M, Cohen H, Cavenagh J, Benjamin S, Starke R, Killick S, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br J Haematol. 2007;136(3):451–61. doi: 10.1111/j.1365-2141.2006.06448.x
- Chemnitz JM, Uener J, Hallek M, Scheid C. Long-term follow-up of idiopathic thrombotic thrombocytopenic purpura treated with rituximab. Ann Hematol. 2010;89(10):1029–33. doi: 10.1007/s00277-010-0968-3
- Froissart A, Buffet M, Veyradier A, Poullin P, Provot F, Malot S, et al. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40(1):104–11. doi: 10.1097/CCM.0b013e31822e9d66
- Pequeño-Luévano M, Villarreal-Martínez L, Jaime-Pérez JC, Gómez-de-León A, Cantú-Rodríguez OG, González-Llano O, et al. Low-dose rituximab for the treatment of acute thrombotic thrombocytopenic purpura: report of four cases. Hematology. 2013;18(4):233–6. doi: 10.1179/1607845412Y.0000000073
- Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118(7):1746–53. doi: 10.1182/blood-2011-03-341131
- Westwood JP, Webster H, McGuckin S, McDonald V, Machin SJ, Scully M. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 2013;11(3):481–90. doi: 10.1111/jth.12114
- McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8(6):1201–8. doi: 10.1111/j.1538-7836.2010.03818.x
- Barcellini W, Zaja F, Zaninoni A, Imperiali FG, Battista ML, Di Bona E, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood. 2012;119(16):3691–7. doi: 10.1182/blood-2011-06-363556
- Zaja F, Vianelli N, Volpetti S, Battista ML, Defina M, Palmieri S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–34. doi: 10.1111/j.1600-0609.2010.01486.x
- Barcellini W, Zaja F, Zaninoni A, Imperiali FG, Di Bona E, Fattizzo B, et al. Sustained response to low-dose rituximab in idiopathic autoimmune hemolytic anemia. Eur J Haematol. 2013;91(6):546–51. doi: 10.1111/ejh.12199
- Zaja F, Battista ML, Pirrotta MT, Palmieri S, Montagna M, Vianelli N, et al. Lower dose rituximab is active in adults patients with idiopathic thrombocytopenic purpura. Haematologica. 2008;93(6):930–3. doi: 10.3324/haematol.12206
- Gómez-Almaguer D, Tarín-Arzaga L, Moreno-Jaime B, Jaime-Pérez JC, Ceballos-López AA, Ruiz-Argüelles GJ, et al. High response rate to low-dose rituximab plus high-dose dexamethasone as frontline therapy in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2013;90(6):494–500. doi: 10.1111/ejh.12102
- Gómez-Almaguer D, Solano-Genesta M, Tarín-Arzaga L, Herrera-Garza JL, Cantú-Rodríguez OG, Gutiérrez-Aguirre CH, et al. Low-dose rituximab and alemtuzumab combination therapy for patients with steroid-refractory autoimmune cytopenias. Blood. 2010;116(23):4783–5. doi: 10.1182/blood-2010-06-291831
- Benjamin M, Terrell DR, Vesely SK, Voskuhl GW, Dezube BJ, Kremer Hovinga JA, et al. Frequency and significance of HIV infection among patients diagnosed with thrombotic thrombocytopenic purpura. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48(8):1129–37.
- Hart D, Sayer R, Miller R, Edwards S, Kelly A, Baglin T, et al. Human immunodeficiency virus associated thrombotic thrombocytopenic purpura – favourable outcome with plasma exchange and prompt initiation of highly active antiretroviral therapy. Br J Haematol. 2011;153(4):515–9. doi: 10.1111/j.1365-2141.2011.08636.x
- Hie M, Gay J, Galicier L, Provot F, Presne C, Poullin P, et al. Preemtive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124(2):204. doi: 10.1182/blood-2014-01-550244
- Lim W, Vesely SK, George JN. The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood. 2015;125(10):1526–31. doi: 10.1182/blood-2014-10-559211