Abstract
Background: Sickle cell disease (SCD) is one of the major health problems in many parts of the world. SCD is characterized by multisystem complications with marked variability in its severity between patients, probably linked to nitric oxide (NO). Endothelial nitric oxide synthase (eNOS) enzyme which is responsible for NO synthesis may be implicated in SCD pathophysiology.
Aim of the study: To explore the possible association between the eNOS gene polymorphisms and severity of SCD. Furthermore, we examined the genomic diversity of these polymorphisms in SCD patients.
Methods: We genotyped 100 SCD patients and 80 controls were genotyped for eNOS 4a/b and eNOS 786T>C polymorphisms, using allele-specific polymerase chain reaction (PCR) and PCR-restriction fragment length polymorphism assay, respectively. Polymorphisms were analyzed in relation to severity of SCD manifestations.
Results: The homozygous mutant eNOS-786T>T genotype was significantly associated with high risk of acute chest syndrome (ACS). The wild-type eNOS-4a/4b genotype was protective against vaso-occlusive crisis (VOC) and pulmonary hypertension (PHTN). The mutant homozygous haplotype (C –4a) was significantly associated with the risk of ACS, VOC, and PHTN.
Conclusion: eNOS intron 4 and eNOS T>C gene polymorphisms may be used as a genetic marker of prognostic value in SCD, as they are associated with unfavorable clinical outcomes.
Background
Sickle cell disease (SCD) is an autosomal-recessive disorder; it is common genetic disease affecting African individuals.Citation1 SCD results from substitution of the normal hydrophilic glutamic acid by a hydrophobic valine. This substitution is due to a single nucleotide mutation (GAG/GTG) in the sixth codon of the beta-globin gene and favors aggregation of hemoglobin molecules.Citation2 As a result, Hb S is less soluble when deoxygenated, precipitates with intraerythrocytic polymerization of deoxyhemoglobin S, and displays a morphologic change to a crescent shape. These rigid sickle cells lead to hemolytic anemia and all complications of SCD.Citation3
SCD is characterized by multisystem complications such as acute chest syndrome (ACS), pulmonary hypertension (PHTN), renal insufficiency, and vaso-occlusive crises (VOCs). SCD complications are characterized by marked variability in severity between individuals.Citation4 The pathophysiology of SCA complications is mainly due to microvascular occlusion by sickled erythrocytes, with subsequent localized tissue ischemia and pain. However, NO deficiency has been considered as a critical factor in causing endothelial dysfunction and vasculopathy that occurs in SCD.Citation5 The impaired NO bioavailability produces many complications such as PHTN, priapism, leg ulcer, stroke, renal insufficiency, esophageal motility disorders and abdominal pain in SCD patients.Citation6
The role of depletion of NO by free hemoglobin in pathogenesis of SCD complication is controversial.Citation7,Citation8 Elevation of plasma hemoglobin levels in SCD patients is modest compared with values obtained in other hemolytic disorders, paroxysmal nocturnal hemoglobinuria in particular.Citation7
Three isoforms of nitric oxide synthase (NOS) enzyme are responsible for NO synthesis during the enzymatic conversion of l-arginine to l-citrulline, including endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS).Citation9
Previous study showed that the eNOS enzyme is responsible for the synthesis of NO that plays critical role in inhibition of both VOC and ACS in sickle cell transgenic murine models. The eNOS enzyme was assigned to be a critical regulator of vasodilation and inhibitor of cell adhesion and aggregation, protecting the animal from vaso-occlusion.Citation10
In human, plasma arginine concentration and NO metabolite levels decreases significantly during VOC and ACS, in both adults and children SCD patients.Citation11 In normal healthy individuals, the allelic variation of the eNOS gene affects the level of plasma NO in the body.Citation12
Published studies suggested association between eNOS gene single-nucleotide polymorphisms and obvious variation in NO levels. These polymorphisms include: eNOS 786T>C (rs2070744) in promoter region, eNOS 894G>T in exon 7 (Glu298Asp, rs1799983), and eNOS 4a/b, a 27-bp variable number of tandem repeats in intron 4 (chromosome 7q35–q36). The allele C of T786C polymorphism was found to decrease promoter activity to less than half of normal activity.Citation13 These eNOS gene polymorphisms have been found to predispose to several vascular disorders such as myocardial infarction,Citation14 renal injury,Citation15 and stroke.Citation16
The eNOS gene polymorphisms may be considered as a potential genetic modifier for the severity of SCD complications. In order, we investigated the possible association between the eNOS 786T>C polymorphism and the eNOS 27-bp repeat polymorphism in intron 4 and severity of SCD complications in the form of VOC, ACS, renal injury, and PHTN in adult SCD patients.
Patients and methods
Clinical and hematologic analysis
We designed a case–control study, including 100 Egyptian SCD patients and 80 Egyptian controls of similar age and sex without significant medical history. The study protocol was in accordance with the local hospital research guidelines. Written informed consents were obtained from all patients before study initiation and patient recruitment. Patients were diagnosed and followed up in the clinical hematology unit of the Kasr Alainy teaching hospital. Clinical as well as demographic data were obtained from medical records and interviews with the patients.
Inclusion criteria of controls include healthy individuals with normal complete blood counts, normal kidney function, normal hemoglobin electrophoresis, and normal echocardiography.
Inclusion criteria of patients include SCD patients who are 18 years or older, without concomitant co-morbidities, such as diabetes mellitus, hypertension, or intrinsic kidney disease. Diagnosis of SCD was based on hemoglobin electrophoresis, and high-performance liquid chromatography (Bio Rad, USA) was used to measure several hemoglobin species (Hb F, Hb A1, Hb A2, and Hb S). SCD patients included in the study did not receive blood transfusion for at least 3 months before isolating the DNA for genotyping.
Patients were followed up prospectively between June 2012 and December 2014 to evaluate the relation between eNOS gene polymorphisms and the frequency of complications of SCD, in the form of number of VOC attacks, ACS, renal injury, and pulmonary hypertension. The average number of VOC per year as well as any attack of ACS was documented. VOC was defined as pain in the extremities, back, and abdomen without any other explanation.Citation17 ACS in SCA is defined as a new infiltrate on chest radiograph associated with one or more symptoms, such as fever, cough, hypoxia, tachypnea, and dyspnea.Citation18 PHTN was evaluated using echocardiography; the diagnosis of PHTN requires right heart catheterization, however, the tricuspid regurgitation jet velocity (TRV) is a useful noninvasive screening tool for suspected PHTN, so we used TRV as a marker for PHTN when TRV > 2.5 m/s.Citation19 The patients were assessed for early renal injury using albumin/creatinine ratio (A/C ratio), as microalbuminuria has been described as a preclinical indicator of glomerular damage in SCD patients.Citation20
Determination of eNOS gene polymorphisms
Blood samples and DNA extraction
Blood samples of 5 ml were obtained from all participants, collected in sterile EDTA tubes, and then stored at −20°C. Genomic DNA of patients and healthy controls was extracted from whole blood using an established protocol for DNA extraction from blood samples using a DNA extraction kit (catalog number 51104; Qiagen, Hilden, Germany). The extracted DNA was amplified by polymerase chain reaction (PCR) using recombinant Taq polymerase master mix (catalog number 201443; Qiagen).
Polymerase chain reaction analysis
PCR reactions were carried out in 25 µl volumes comprising 12.5 µl Taq PCR master mix(0.1 units/µL Taq DNA polymerase, 32 mM (NH4)2 SO4, 130 mM Tris–HCl, 5.5 mM MgCl2, and 0.4 mM of each dNTP), 5.5 µl distilled water, 5 µl template DNA, and 1 µl of each primer. Primers were synthesized by Sangon and prepared to a concentration of 25 pmol each. For eNOS gene 27-bp repeat polymorphism in intron 4, the following primers were used: forward primer: 5′-AGG CCC TAT GGT AGT GCC TTT-3′; reverse primer: 5′-GCC TCC ACC CCC ACC CTG TC-3′. For eNOS T-786C polymorphism, the following primers were used: forward primer: 5′-TGG AGA GTG CTG GTG TAC CCC A-3′; reverse primer: 5′-GCC TCC ACC CCC ACC CTG TC-3′. The PCR amplifications were carried out using the thermal cycler Applied Biosystems 9600 (Perkin Elmer). Main cycling parameters were 94°C for 4 min for initial denaturation, followed by 35 cycles of denaturation at 94°C for 30 s and annealing at 63°C for 30 s with final extension was at 72°C for 5 min. The PCR product was run in 4% agarose gel.
Detection of eNOS gene 27-bp repeat polymorphism in intron 4
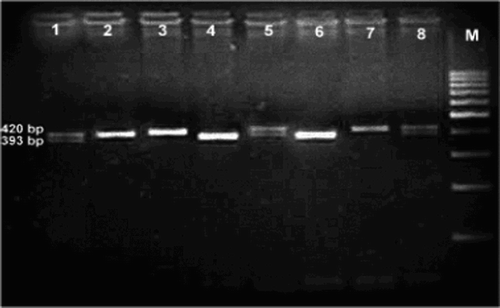
The 27 bp repeat VNTR in intron 4 (eNOS 4a/b) was differentiated by allele-specific PCR. Mutant allele eNOS 4a (four repeats) produced 393 bp fragment and wild-type allele eNOS 4b (five repeats) produced 420 bp fragment; therefore, the wild homozygous genotype (b/b) will appear as a single band of 420 bp, while the homomutant genotype (a/a) will appear as a single band of 393 bp, and the heteromutant genotype (b/a) will appear as two bands at 420 and 393 bp (Fig. ).
Detection of eNOS T-786C genetic polymorphism
The T→C transition at position −786 in the 5′ flanking region of the eNOS gene was determined by PCR-restriction fragment length polymorphism analysis. The wild-type allele (T allele) of the amplified fragment contains one restriction site that can be recognized by the restriction enzyme MspI leading to digestion of the 180 bp fragment into two fragments of 140 and 40 bp, while the polymorphic allele (C allele) contains two restriction sites that can be recognized by the restriction enzyme leading to its digestion into three fragments of 90, 50, and 40 bp. The wild homozygous genotype (TT) appears as two bands (140 and 40 bp), while the mutated homozygous genotype (CC) appears as three bands (90, 50, and 40 bp) and the mutated heterozygous genotype (TC) appear as four bands (140, 90, 50, and 40 bp; Fig. ).
Figure 2 Genotyping of eNOS T-786C polymorphism in the promoter region. Lanes 1, 3, and 7 show 140 bp bands, denoting wild type (T) allele. Lanes 5, 6, and 8 show 90 bp bands, denoting homomutant type (C) allele. Lanes 2 and 4 show bands at 140 and 90 bp, denoting heteromutant type (TC). M is a marker ladder. NB: Bands at 50 and 40 bp could not be seen.

Statistical analysis
The data were coded and entered using the statistical package SPSS version 15. The data were summarized using descriptive statistics: mean, standard deviation, median and interquartile range for quantitative variables, and number and percentage for qualitative values. Statistical differences between groups were tested using chi-square test for qualitative variables, while nonparametric Mann–Whitney test and Kruskal–Wallis test were used for quantitative variables, which are not normally distributed. Logistic regression analysis was done to test for significant predictors of dependent variables. P-values ≤0.05 were considered statistically significant.
Results
One hundred SCD patients were enrolled in this study, 47 patients (47%) were females and 53 patients (53%) were males. Patients' age ranged from 18 to 34 years, and the mean of the patients' age was 24.6 ± 3.4 years. Out of the 100 patients, 39 patients (39%) were diagnosed as sickle beta thalassemia (Hb SB), while 61 patients (61%) were diagnosed as sickle cell anemia (Hb SS).
Eighty healthy controls were enrolled in this study: among which 45 controls (56.25%) were males and 35 (43.75%) were females. Controls' age ranged from 20 to 33 years, and the mean of the controls' age was 26.3 ± 2.5 years.
Out of 100 patients, 69 patients (69%) had attacks of VOC, while 31 patients (31%) had no attacks. The number of VOC per year ranged from 1 to 6 attacks/year; the median was 2 attacks/year. ACS was observed in 16 patients (16%), while 84 patients (84%) had no attack of ACS. PHTN was observed by echocardiography in 32 patients (32%); 68 patients (68%) had no pulmonary hypertension. Among the patients enrolled in this study, the A/C ratio ranged from 0.01 to 4; the median was 0.32.
The frequency distribution of the eNOS –786T>C polymorphism in the studied individuals revealed the highest frequency was for the heterozygous mutant genotype (TC), followed by the wild-type genotype (TT), and then the homozygous mutant genotype (CC). However, there was no statistically significant difference between patients and controls (49 and 47.5% had CT genotype, 39 and 41.3% had TT genotype, and 12 and 11.3% had CC genotype of patients and controls, respectively, P-value = 0.759, 0.841, and 0.876 for TC, TT, and CC genotypes, respectively; Table ).
Table 1 Distribution of eNOS genotypes among SCD patients and the controls
The frequency distribution of the eNOS 4a/b polymorphism in the studied individuals revealed the highest frequency was for the wild-type genotype (bb), followed by the heterozygous mutant genotype (ab), and then the homozygous mutant genotype (aa). However, there was no statistically significant difference between patients and controls (52 and 62.5% had bb genotype, 32 and 25% had ab genotype, and 16 and 12.5% had aa genotype of patients and controls, respectively, P-value = 0.158, 0.303, and 0.507 for bb, ab, and aa genotype, respectively; Table ).
The frequency distribution of the four eNOS haplotypes was compared between patients and controls. Although the homozygous mutant haplotype (786 C-4a) was the most frequent haplotype among SCD patients (34, 27, 14, and 25% of the patient were carrying the 786 C-4a haplotype, 786 C-4b haplotype, 786 T-4a haplotype, and 786 T-4b haplotype, respectively), there was no statistically significant difference when compared with controls. P-value = 0.572, 0.769, 0.326, and 0.190 for 786 C-4a haplotype, 786 C-4b haplotype 786 T-4a haplotype, and 786 T-4b haplotype, respectively (28.8, 12.5, 33.8, and 25% of the controls were carrying the 786 C-4a haplotype, 786 C-4b haplotype 786 T-4a haplotype, and 786 T-4b haplotype respectively; Table ).
Table 2 Distribution of eNOS haplotypes among SCD patients and controls
Among SCD patients, as there was statistically significant gender-wise difference in frequency distribution of eNOS 786T>C and eNOS 4a/b genotypes, the wild genotypes were statistically significantly higher in female patients when compared with males (For eNOS 786T>C polymorphism, the TT genotype was observed in 51.1 vs. 28.3% of female and males respectively, P-value = 0.02. For eNOS 4a/b polymorphism, the bb genotype was observed in 66.0 vs. 39.6% of females and males, respectively, P-value = 0.009). The heterozygous genotypes were significantly higher in male patients when compared to females (for eNOS 786T>C polymorphism: TC genotype was observed in 21.2 vs. 41.5% of females and males, respectively, with P-value = 0.044. For eNOS 4a/b polymorphism, bb genotype was observed in 59.6 vs. 40.4% of females and males, respectively, P-value = 0.030) (Table ).
Table 3 Comparison between SCD patients carrying various eNOS genotypes according to gender
The genotypes frequency distribution was compared between SCD patients with and without ACS (ACS+ and ACS–), VOC attacks (VOC+ and VOC–), and PHTN (PHTN+ and PHTN–). There was statistically significant difference in the frequency distribution of eNOS 786T>C polymorphism between ACS+ and ACS– groups; the CC genotype was significantly associated with occurrence of ACS (43.75, 31.25, and 25% of ACS+ patients were carrying the CC, TC, and TT genotype, respectively, while 5.95, 52.38, and 41.66% of ACS– patients were carrying the CC, TC, and TT genotype, respectively, P-value = 0.000, 0.121, and 0.210 for CC, TC, and TT genotype, respectively). There was no statistically significant difference in the frequency distribution of the eNOS 4a/b polymorphism between ACS+ and ACS– groups (Table ).
Table 4 Comparison between various eNOS genotypes according to presence and absence of SCD manifestations (VOC, ACS, and PHTN)
There was also statistically significant difference in the frequency distribution of the eNOS 4a/b polymorphism between VOC+ and VOC– groups; the wild-type genotype (bb) was significantly associated with low risk of VOC compared to other genotypes (18.84, 39.13, and 42.02% of VOC+ patients were carrying the aa, ab and bb genotype, while 9.67, 16.12, and 74.19% of VOC– patients were carrying the aa, ab, and bb genotype, respectively; P-value = 0.248, 0.230, and 0.003 for aa, ab, and bb genotype, respectively). There was no statistically significant difference in the frequency distribution of the eNOS 786T>C polymorphism between VOC+ and VOC– groups (Table ). Logistic regression analysis was done to test significant predictors of ACS; sex, age, eNOS 4a/b, and eNOS 786T>C polymorphisms were used in regression model. The eNOS 786T>C polymorphism was found to be the only significant predictor of ACS; patients carrying the homozygous mutant genotype (CC) had about 10 times risk to develop ACS compared with wild-type genotype (TT) (P-value = 0.007, odds ratio = 10.091).
There was statistically significant difference in the frequency distribution of the eNOS 4a/b polymorphism between PHTN+ and PHTN– groups; the wild-type genotype was significantly associated with low risk of PHTN compared to other genotypes (18.75, 37.5, and 43.75% of PHTN+ patients were carrying the aa, ab, and bb genotypes, while 14.70, 26.47, and 58.82% of PHTN– patients were carrying the aa, ab, and bb genotypes, respectively; P-value = 0.607, 0.084, and 0.046 for aa, ab, and bb genotypes, respectively. There was no statistically significant difference in the frequency distribution of eNOS 786T>C polymorphism between PHTN+ and PHTN– groups of patients (Table ).
The haplotypes frequency distribution was compared between SCD patients with and without ACS (ACS+ and ACS–), VOC attacks (VOC+, VOC–), and PHTN (PHTN+ and PHTN–). There was statistically significant difference in the frequency distribution of the 4 haplotypes among patients with and without VOC, ACS, and PHTN; frequencies of VOC, ACS, and PHTN were statistically significant higher in patients carrying the homozygous mutant haplotype 4 (eNOS 786 C – eNOS 4a) compared to other haplotypes (P-value = 0.003, 0.040, and 0.021 for VOC, ACS, and PHTN, respectively) (for more details, see Table ).
Table 5 Comparison between various eNOS haplotypes (eNOS 786T>C – eNOS 4a/b) according to presence and absence of SCD manifestations (VOC, ACS, and PHTN)
Patients were stratified according to genotype/haplotype, and then the mean and median of the number of VOC, as well as A/C ratio were compared. The number of VOC was statistically significant higher in patients carrying the mutant eNOS 4a/b genotypes (mean of VOC was 2.31, 2.19, and 1.66 for patients carrying the aa, ab, and bb genotype, respectively, P-value = 0.011). However, the mean of the A/C ratio was not statistically significant different between various eNOS 4a/b genotypes. Regarding eNOS 786C>T polymorphism, the mean of the number of VOC and A/C ratio was not statistically significant different between patients carrying various eNOS 786C>T genotypes (Table ).
Table 6 Comparison between eNOS (eNOS 786T>C – eNOS 4a/b) genotypes according to number of VOC and A/C ratio
The number of VOC was statistically significantly higher in patients carrying the homozygous mutant haplotype 4 (eNOS 786 C – eNOS 4a) (mean of VOC was 1.4 ± 0.63, 2.1 ± 1.29, 1.93 ± 1.77, and 2.27 ± 1.11 for patients carrying the 786 C-4a haplotype, 786 C-4b haplotype 786 T-4a haplotype, and 786 T-4b haplotype, respectively, P-value = 0.02). However, the mean and the median of A/C ratio were not statistically significantly different between patients carrying various eNOS haplotypes (Table ).
Table 7 Comparison between eNOS (eNOS 786T>C – eNOS 4a/b) haplotypes according to number of VOC and A/C ratios
Discussion
The diversity of severity of SCD symptoms is challenging extensive studies aiming at understanding the possible genetic factors modulating the severity of SCD. The eNOS gene polymorphisms (eNOS 4a/b, eNOS 894G>T, and eNOS 786T>C) play a pivotal role in eNOS gene expression and subsequently serum level of NO in SCD patients.Citation12,Citation21
Obvious relation between eNOS gene polymorphisms and vasculopathy had been found in various diseases.Citation22–Citation25 Therefore, the present study aimed at exploring possible association of eNOS gene polymorphisms and severity of SCD manifestations in Egyptian SCD patients, as eNOS gene polymorphisms may be considered as a potential genetic modifier in SCD. Furthermore, we examined the genomic diversity and haplotype frequency of eNOS gene polymorphisms in the Egyptian individuals.
Among the studied individuals, the frequency distribution of eNOS –786T> C and eNOS 4a/b polymorphisms was similar among patients and controls. The homozygous mutant haplotype (eNOS 786C– eNOS 4a/4a) was the most frequently observed haplotype among our SCD patients; however, there was no statistically significant difference when compared to controls. We reported no association between eNOS polymorphisms and SCD.
There was gender-wise difference in the frequency distribution of eNOS polymorphisms; female sex was associated with the wild-type genotype of both polymorphisms.
Tantawy et al. studied the prevalence of eNOs 4a/b polymorphisms in 51 Egyptian patients with SCD compared with 55 healthy controls. Similar to our results, they reported highest frequency was for wild-type genotype, with no difference in the distribution of eNOS genotypes between SCD patients and controls.Citation26
Thakur et al. studied the prevalence of eNOS polymorphisms (T786C, G894T, and 4a/b) in SCD patients and controls recruited from Mali. They reported that the wild-type alleles were the most frequent for all eNOS polymorphisms between cases and controls.Citation27 Unlike our results, the frequency of studied haplotypes showed the highest frequency was for haplotype combined with the wild-type homozygotes for eNOS −786T >C and eNOS 4a/b with no significant difference in haplotypes frequency distribution between cases and controls. Our result is supported by a Brazilian study, which reported no association between SCD and eNOS variants.Citation28
However, Nishank et al. reported a higher prevalence of mutant alleles, genotypes, and haplotypes of the three eNOS polymorphisms (–eNOS 4a/b, eNOS 894G>T, and eNOS –786T>C) in SCD individuals, suggesting an association between eNOS gene polymorphisms and SCD in India.Citation22 Unlike our results, they reported no gender-wise differences in allelic and genotypic distribution. These different results may be explained by extensive interethnic diversity of eNOS gene polymorphisms.
In our study, the wild-type eNOS 4a/4b genotype (bb) was protective against VOC and PHTN. Our results were supported by Tantawy et al. observations, as they reported higher frequency of VOC and PHTN in patients carrying the eNOS4-a allele.Citation26 We also observed that the mutant homozygous haplotype (eNOS 786C –eNOS 4a) was also significantly associated with ACS, VOC, and PHTN.
Chaar et al. studied the association of eNOS gene polymorphisms (4a/b and T-786C respectively), and the occurrence of ACS and VOC in 173 SCD children in Europe. They reported no association between the studied polymorphisms and VOC.Citation29
We observed that the homozygous mutant eNOS 786T>C (CC) genotype was significantly associated with occurrence of ACS. Similar to our results, Sharan et al. reported association between the eNOS C-786 allele in African-American SCD patients and increased susceptibility to ACS, but this observation was assigned to female patients only.Citation30 However, surprisingly Chaar et al. reported that the mutant eNOS C-786 allele decreases the risk of ACS in pediatric patients. They explained it by age-related differences and other clinical events that are considered to play a significant pathogenic role in ACS.Citation29
Impaired NO bioavailability represents the central feature of endothelial dysfunction, and pervasively contributes to the overlapping mechanisms of vasculopathy in SCD specially ACS and VOC.Citation9 The homozygous mutant (CC) genotype of eNOS 786T>C polymorphism results in low levels of NO as it decreases eNOS activityCitation31; in our study, we assigned eNOS –786 (CC) genotype as independent risk factor for ACS.
Although we found no association between eNOS gene polymorphisms and renal injury, Tantawy et al. reported significant relation between eNOS 4a/b polymorphism and degree of nephropathy (micro-/macroalbuminuria).Citation26 Our different results may be explained by age-related variation of renal injury mechanisms in SCD patients, as our study population was adult. This may suggest a minor role of NO bioavailability in the pathogenesis of nephropathy in adult.
Unlike renal injury, we found a strong association between eNOS polymorphisms and risk of ACS, PHTN, and VOC in adults as in children, suggesting the critical role of NO bioavailability in pathogenesis of vasculopathy regardless of the age of SCD patients.
Conclusion
Impairment of NO bioavailability plays a critical role in the pathogenesis of SCD vasculopathy. As the 27-bp repeat polymorphism in intron 4 and –786T>C polymorphism of eNOS gene are associated with unfavorable clinical outcomes, eNOS gene polymorphisms may be used as a genetic marker of prognostic value for defining patients at risk of developing severe SCD complications due to impaired NO bioavailability; these patients may get benefits of using treatments aimed at enhancing arginine and NO bioavailability.
Disclaimer statements
Contributors Sherif M. Yousri and Gehan H. Shahin performed the conception of the study, all the laboratory work up, and revised the article; Hend N. Ellithy designed the research and analyzed data, discussed the final results, and wrote the paper. All authors read and approved the final version of the manuscript.
Funding None.
Conflicts of interest The authors declared no conflicts of interest.
Ethics approval The study protocol was in accordance with the local hospital research guidelines.
Acknowledgments
We are grateful to all patients and controls enrolled in this study.
References
- Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010;115:4331–6. doi: 10.1182/blood-2010-01-251348
- Bunn HF. Sickle hemoglobin and other hemoglobin mutants. In: Stamatoyannopoulos G, Nienhuis AW, Majerus PW, Varmus H, (eds.) The molecular basis of blood diseases. Philadelphia: WB Saunders; 1994. p. 207–56.
- Serjeant GR, Serjeant BE, Mohan JS, Clare A. Leg ulceration in sickle cell disease: medieval medicine in a modern world. Hematol Oncol Clin North Am. 2005;19:943–56. doi: 10.1016/j.hoc.2005.08.005
- Serjeant GR. Sickle cell disease. 2nd ed. Oxford: Oxford University Press; 1992.
- Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radical Biol Med. 2008;44:1506–28. doi: 10.1016/j.freeradbiomed.2008.01.008
- Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130(6):943–53. doi: 10.1111/j.1365-2141.2005.05701.x
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477
- Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 2010;116(5):687–92. doi: 10.1182/blood-2010-02-268193
- Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–9. doi: 10.1242/jcs.01165
- Pritchard Jr. KA, Ou J, Ou Z, Shi Y, Franciosi JP, Signorino P, et al. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:705L–14. doi: 10.1152/ajplung.00288.2002
- Morris CR, Kuypers FA, Larkin S, Vichinsky E, Styles L. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000;22:515–20. doi: 10.1097/00043426-200011000-00009
- Tsukada T, Yokoyama K, Aria T, Takemoto F, Hara S, Yamada A, et al. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophysics Res Commun. 1998;245:190–3. doi: 10.1006/bbrc.1998.8267
- Kunnas TA, Ilveskoski E, Niskakangas T, Laippala P, Kajander OA, Mikkelsson J, et al. Association of the endothelial nitric oxide synthase gene polymorphism with risk of coronary artery disease and myocardial infarction in middle-aged men. J Mol Med. 2002;80:605–9. doi: 10.1007/s00109-002-0365-z
- Wang XL, Wang J. Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol Genet Metab. 2000;70:241–51. doi: 10.1006/mgme.2000.3033
- Nagase S, Suzuki H, Wang Y, Kikuchi S, Hirayama A, Ueda A, et al. Association of ecNOS gene polymorphisms with end stage renal diseases. Mol Cell Biochem. 2003;244(1–2):113–8. PMID: 12701818 doi: 10.1023/A:1022473405275
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–9. doi: 10.1016/j.tins.2004.03.009
- Tarer V, Etienne-Julan M, Diara JP, Belloy MS, Mukizi-Mukaza M, Elion J, et al. Sickle cell anemia in Guadeloupean children: pattern and prevalence of acute clinical events. Eur J Haematol. 2006;76:193–9. doi: 10.1111/j.1600-0609.2005.00590.x
- Ballas SK, Lieff S, Benjamin LJ, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85:6–13. doi: 10.1002/ajh.21750
- Ambrusko SJ, Gunawardena S, Sakara A, Windsor B, Lanford L, Michelson P, et al. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):907–13. doi: 10.1002/pbc.20791
- Guash A, Cua M, Mitch WE. Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int. 1996;49:686–91.
- Nishank SS, Singh MP, Yadav R, Gupta RB, Gadge VS, Gwal A. Endothelial nitric oxide synthase gene polymorphism is associated with sickle cell disease patients in India. J Hum Genet. 2013;58:775–9. doi: 10.1038/jhg.2013.99
- Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with tartrate vs. glutamate at position 298. Proc Natl Acad Sci USA. 2000;97:2832–5. doi: 10.1073/pnas.97.6.2832
- Liu R, Geng P, Ma M, Yu S, Wang X, Zhang W, et al. Association between endothelial nitric oxide synthase gene polymorphism (T-786C) and ischemic stroke susceptibility: a meta-analysis. Int J Neurosci. 2014;124(9):642–51. doi: 10.3109/00207454.2013.873978
- Ekmekçi A, Ozcan KS, Güngör B, Abaci N, Osmonov D, Zencirci A, et al. The relationship between endothelial nitric oxide synthase 4a/4b gene polymorphism and premature coronary artery disease. Acta Cardiol. 2013;68:464–8.
- Tantawy AA, Adly AA, Ismail EA, Aly SH. Endothelial nitric oxide synthase gene intron 4 VNTR polymorphism in sickle cell disease: relation to vasculopathy and disease severity. Pediatr Blood Cancer. 2015;62(3):389–94. doi: 10.1002/pbc.25234
- Thakur TJ, Guindo A, Cullifer LR, Li Y, Imumorin IG, Diallo DA, et al. Endothelin-1 but not endothelial nitric oxide synthase gene polymorphism is associated with sickle cell disease in Africa. Gene Regul Syst Biol. 2014;8:119–26. doi:10.4137/GRSB.S14836
- Vargas AE, da Silva MAL, Silla L, Chies JAB. Polymorphisms of chemokine receptors and eNOS in Brazilian patients with sickle cell disease. Tissue Antigens. 2005;66:683–90. doi: 10.1111/j.1399-0039.2005.00506.x
- Sharan K, Surrey S, Ballas S, Borowski M, Devoto M, Wang KF, et al. Association of T-786C eNOS gene polymorphism with increased susceptibility to acute chest syndrome in females with sickle cell disease. Br J Hematol. 2004;124:240–3. doi: 10.1046/j.1365-2141.2003.04762.x
- Chaar V, Tarer V, Etienne-Julan M, Diara JP, Elion J, Romana M. ET-1 and ecNOS gene polymorphisms and susceptibility to acute chest syndrome and painful vaso-occlusive crises in children with sickle cell anemia. Haematologica. 2006;91(9):1277–8.
- Mack AK, Kato GJ. Sickle cell disease and nitric oxide: a paradigm shift? Int J Biochem Cell Biol. 2006;38:1237–43. doi: 10.1016/j.biocel.2006.01.010
- Elshamaa MF, Sabry S, Badr A, El-Ahmady M, Elghoroury EA, Thabet EH, et al. Endothelial nitric oxide synthase gene intron 4 VNTR polymorphism in patients with chronic kidney disease. Blood Coagul Fibrinolysis. 2011;22:487–92. doi: 10.1097/MBC.0b013e328346ef71