Abstract
Introduction: The increased flow cytometry enumeration of peripheral blood circulating CD34+ cells in patients with acute leukemia has been found in our previous work. In this study, we also demonstrated that acute promyelocytic leukemia (APL) patients not only had elevated CD34+ cell count, but also had some clinical features.
Methods: Fifty APL patients and 19 healthy volunteers were included in the study. The enumeration of circulating CD34+ cells, cytogenetic subgroup, immunophenotype analysis, and leukemic-related gene mutation detection were performed.
Results: Some APL patients with higher count of CD34+ cells (≤10 × 106/l) usually possessed one or more poor prognostic factors (higher WBCs count, PML/RARa gene complex fusion, chemotherapy-related APL, normal karyotype/complex karyotype abnormalities, CD56/CD34 antigen positive expression, FLT3-ITD positive mutation, myelofibrosis, and marrow necrosis). A cut-off value of 10 × 106/l CD34+ cells may have the power to distinguish APL patients with above adverse clinical prognostic factor from other APL subjects.
Conclusion: The circulating CD34+ cell count appears to increase in some APL patients and a higher CD34+ cell count may be indicative of inferior survival and serve as an adverse biomarker for APL.
Introduction
Acute promyelocytic leukemia (APL) is a subtype of myeloid leukemia which is characterized by leukemic cells blocked at the promyelocytic stage of granulocytic differentiation and is associated with the presence of balanced t(15;17) and PML/RARa fusion transcripts.Citation1 CD34 antigen is transmembrane glycophosphoprotein expressed on early hematopoietic cells,Citation2 in steady state, there is little exchange of hematopoietic stem cells from the marrow through the blood and into the marrow under basal conditions, while many factors can facilitate bone marrow CD34+ cells influx into peripheral blood.Citation3
Previous studies have reported that increased peripheral blood circulating CD34+ cell enumeration in patients with Myelodysplastic syndrome (MDS), idiopathic myelofibrosis (IMF) and chronic myeloid leukemia (CML) correlated with disease grade-related and leukemia-free survival, and have found its usefulness as a clinical marker of disease prognosis.Citation4–Citation7 As for patients with acute leukemia (AL), foreign co-workers and our study have similar results, namely, markedly elevated count of CD34+ cells and the level of CD34+ cells could fluctuate in accordance with tumor burden. Significantly correlation was found between the level of CD34+ cells and WBCs count, BM blast CD34 antigen expression intensity.Citation4,Citation8,Citation9 Considering the fact that APL patients usually had BM promyelocyte CD34 antigen negative expression, we estimated that the CD34+ cells count was not higher.Citation10 Interestingly, with our further work, we found APL patients not only had elevated CD34+ cell count, but also had some clinical features. Our aim in the present study was to fully investigate the enumeration of CD34+ cells in APL patients.
Patients and methods
Patients
Between February 2011 and June 2015, peripheral blood samples from 50 patients with APL (22 males and 28 females; median age, 45 years; age range, 18–85 years) and 19 age-adjusted healthy volunteers (11 males and eight females; median age, 31 years; age range, 22–38 years) were recruited. Diagnoses of all patients were made according to World Health Organization (WHO) criteria.
Methods
Enumeration of circulating CD34+ cells
The peripheral blood samples were drawn into EDTA anticoagulated tubes for complete blood count and enumeration of CD34+ cells by flow cytometry. Cells (50 μl of blood) were incubated for 15 minutes at room temperature with fluorescein isothiocyanate-conjugated CD45 monoclonal antibody (Becton Dickinson Immunocytometry Systems) and phycoerythrin (PE)-conjugated CD34 monoclonal antibody (Becton Dickinson) or PE-conjugated isotype matched mouse IgG1 (Becton Dickinson) which was chosen as the negative control. FACS Lysing Solution (Becton Dickinson) was then added to lyse the red blood cells (RBCs). After a wash, the cells were resuspended in PBS and analyses were performed by FACS Calibur flow cytometer (Becton Dickinson).The cell Quest Pro COUNT program was used to acquire CD34+ cells.
Cytogenetic analysis
Bone marrow samples for cytogenetic analyses were performed after short-term culture (24 or 48 hours) following standard procedures. The chromosomes were stained by G-banding and the karyotypes were reported according to International System for Human Cytogenetic Nomenclature (ISCN, 1995) recommendation. Based on the cytogenetic analyses, the subgroup was made as follows: (1) classical chromosome translocation, namely t(15;17); (2) non-classical chromosome translocation, including complex karyotype abnormalities (≥3 abnormalities), normal karyotype and +8 cytogenetic aberrations.
Immunophenotype analysis
Cell differentiation antigen was determined by direct immunofluorescence staining, four-color flow cytometry using conventional gating strategies was used to immunophenotype CD34+ cells in bone marrow nucleated cells. ≥20% was positive.
Leukemic-related gene mutation detection
The bone marrow (2 ml) was drawn into EDTA anticoagulated tubes, the gene mutation of FLT3-ITD, PML/RARα and other leukemic-related gene mutation were determined by polymerase chain reaction.
Subgroups of prognosis and treatment
Low-risk patients had a WBCs count less than 10 × 109/l and had a platelet count more than 40 × 109/l; intermediate-risk patients had a WBCs count less than 10 × 109/l and a platelet count less than 40 × 109/l; and high-risk patients had a WBCs count equal to or more than10 × 109/l. All patients received induction, consolidation and maintenance treatment according to guidelines set by the Hematological Society of the Chinese Medical Association. All samples were collected after obtaining informed consents.
Once a diagnosis of APL was suspected, all-transretinoic acid (ATRA) 20 mg/m2/day was given as induction treatment, as early as possible, until complete remission (CR) was achieve. Among 11 ‘high-risk’ APL patients, nine patients received ATRA, arsenic trioxide (0.16 mg/kg/day, until CR) and anthracycline-based chemotherapy (idarubicin 8 mg/m2/day or daunorubicin 45 mg/m2/day on days 2,4, and 6), one patient was given ATRA and arsenic trioxide treatment because of pulmonary infection, one patient received ATRA and died from pneumorrhagia. Of all 39 ‘intermediate-low risk’ APL patients, 23 patients experienced ATRA and anthracycline-based chemotherapy (of whom one patient died from gastrointestinal hemorrhage), 10 patients were given ATRA and arsenic trioxide because of infection or elderly age, five patients received ATRA, arsenic trioxide, and anthracycline-based chemotherapy, one patient was given ATRA and died from brain hemorrhage. Remission induction was followed by three consolidation cycles with anthracycline-based regimens. Maintenance treatment continued for 2 years and comprised at least five cycles of 3 months each. Each cycle comprised intermittent ATRA (20 mg/m2/day for 14 days), arsenic trioxide (0.16 mg/kg/day for 14 days), and continuous oral methotrexate (15 mg/m2 qw for 4 weeks), combined or not with 6-mercaptopurine (50 mg/m2/day for 2–4 weeks).
Statistical methods
By SAS Statistical software, Multiple Linear Regression was employed, differences in distribution of variables among subsets of patients were analyzed using Fisher's exact tests. Survival curves were drawn using the Kaplan–Meier estimate and compared by the log-rank tests. P-values less than 0.05 were considered statistically significant.
Results
The expression patterns of CD34+ cells among the patients and normal subjects
The median percentage and median absolute count of peripheral blood circulating CD34+ cells in normal subjects were 0.04% (range, 0.01–0.14%), 1.85 × 106/l (range, 0.9–4.69 × 106/l), the numbers of CD34+ cells in APL patients were respectively 0.08% (range,0.02–60.36%), 3.84 × 106/l (range, 0.15–5130.6 × 106/l). Among APL group, ‘high-risk’ APL patients had median CD34+ levels of 0.08% (range, 0.02–18.62%), 15.76 × 106/l (range, 4.9–4133.6 × 106/l), the median values of CD34+ cells in ‘intermediate-low risk’ APL patients were 0.08% (range, 0.02–60.36%), 1.13 × 106/l (range, 0.15–5130.6 × 106/l).
APL patients had significantly higher absolute count of CD34+ cells, compared with normal subjects (P = 0.020, K–S test), also significant difference of the median percentage of circulating CD34+ cells was found between normal subjects and APL patients (P = 0.014, K–S test). There was no obvious difference of the percentage of CD34+ cells between ‘high-risk’ APL patients and ‘intermediate-low risk’ APL patients (P = 0.841, Mann–Whitney Test), but the absolute count of CD34+ cells of ‘high-risk’ APL patients was significantly higher than ‘intermediate-low risk’ APL patients (P = 0.005, Mann–Whitney Test). Compared with normal subjects, ‘high-risk’ APL patients had obviously increased circulating CD34+ cell percentage and count (P = 0.020, P < 0.001, Mann–Whitney Test), but the absolute count of CD34 + cells of ‘intermediate-low risk’ APL patients and normal subjects was close (P = 0.613), while the percentage of circulating CD34+ cells of ‘intermediate-low risk’ APL patients was higher than normal subjects (P = 0.006). For comparison, all normal subjects had absolute count of CD34+ cells lower than 10 × 106/l (Fig. )
Figure 1 Each circle or square or triangle represents one study and the bars represent the median count of each subgroup. APL compared with normal, P = 0.020; high compared with intermediate-low, P = 0.005; intermediate-low compared with normal, P = 0.613; high compared with normal P < 0.001.
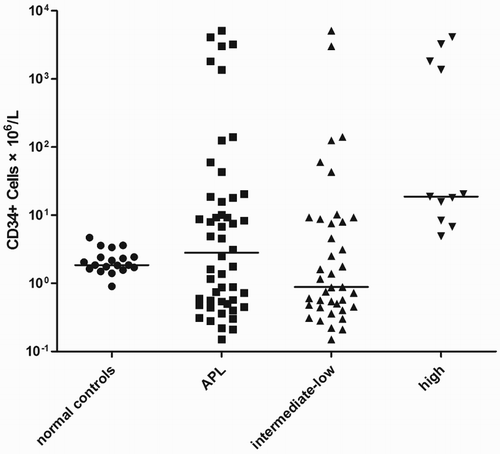
Among 11 ‘high-risk’ APL patients, eight patients had absolute value of CD34+ cells higher than 10 × 106/l, of all 39 ‘intermediate-low risk’ APL patients, only seven patients exceeded the level of 10 × 106/l. In contrast to ‘intermediate-low risk’ APL subgroup, more patients whose CD34+ cell absolute values were above 10 × 106/l appeared in ‘high-risk’ APL subgroup (P = 0.001), and the difference of absolute counts of CD34+ cells between two subgroups was marked (P = 0.005) (Fig. )
The main characteristics of the patients according to CD34+ cell count
There was no significant correlation between circulating CD34+ cell percentage and absolute value and the following continuous parameters in the studied cohort: patients’ age (P = 0.700, P = 0.750), sex (P = 0.360, P = 0.500), BM promyelocyte percentage (P = 0.360, P = 0.350) in APL group. However, the absolute value seemed to be correlated to WBCs count (P = 0.008), while the percentage did not (P = 0.460). CD34+ cell absolute count above 10 × 106/l was observed in 15 patients, of particular interest were clinical features of these patients, namely: (i) eight patients with non-classical chromosomal translocation (four with complex karyotype abnormalities/one with normal karyotype/two with t(15;17) translocation and alone trisomy 8/one with t(15;17) translocation and t(7;19) translocation); (ii) four patients with FLT3-ITD positive mutation; (iii) four patients with BM promyelocyte CD56/CD34 antigen positive expression (one with CD34+CD56+, two patient with CD34+CD56−, one patient with CD34−CD56+); (iv) one patient with myelofibrosis and marrow necrosis; (v) one patient with chemotherapy-related APL; (vi) one patient with PML/RARa gene complex fusion; (vii) eight patients with WBCs count >10 × 109/l (Table and Fig. ).
Figure 2 Each circle or filled circle represents one study. Circle represents poor clinical indexes, respectively, WBCs count >10 × 109/l, FLT3-ITD positive mutation, PML/RARa gene complex fusion, Myelofibrosis and marrow necrosis, Chemotherapy-related APL, CD56/CD34 antigen positive expression, non-classical chromosomal translocation
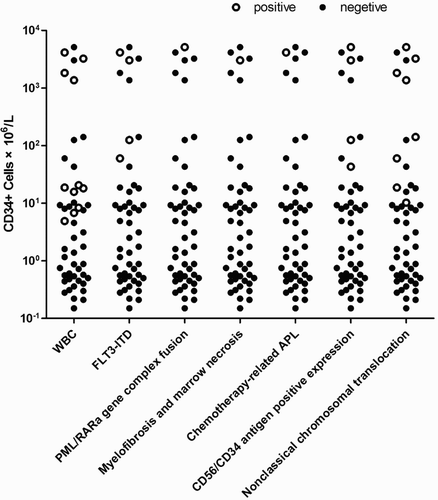
Table 1 Clinical characteristics of 15 APL patients with elevated CD34+ cell counts
Association between CD34+ cell count and remission or survival
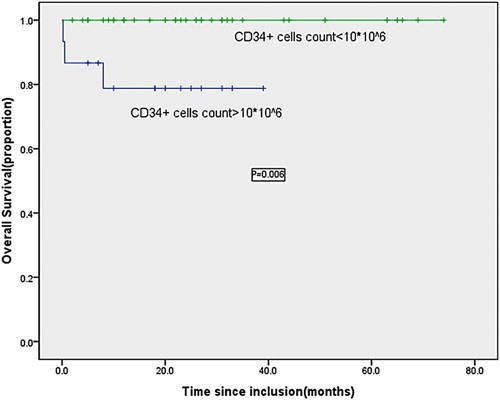
By the follow-up, three (20.0%) of the 15 patients with higher numbers of CD34+ cells had died early (namely No. 1, No. 2, and No. 3 patient, No. 1 and No. 3 patient died from brain hemorrhage and pneumorrhagia, respectively, No. 2 patient died from gastrointestinal hemorrhage), correspondingly the counts of CD34+ cells in three early deaths were 3031.3 × 106/l (No. 1 patient), 5130.6 × 106/l (No. 2 patient) and1362.5 × 106/l (No. 3 patient), significantly elevated counts compared with normal level. The remaining 12 patients and other 35 subjects with normal level of CD34+ cells received complete cytological remission after treatment, and the absolute counts of CD34+ cells in all patients reached below 10 × 106/l at the same time. CR rate was respectively 80% and 100% in higher CD34+ cell subgroup and in normal subgroup, significant difference was observed (P = 0.023) . Follow-ups of CD34+ cell count were available from all 47 patients receiving regular therapy. With a median follow-up of 24 months (range, 2–74 months), all patients were in complete molecular remission, the counts of CD34+ cells in all patients were less than 10 × 106/l. The 5-year overall survival (OS) in subgroup with higher CD34+ cell count was significantly lower than that in normal subgroup with CD34+ cell count (P < 0.01) (Fig. ).
Discussion
APL is a special subtype in acute myeloid leukemia, which can be divided into ‘high risk’ subgroup and ‘intermediate-low risk’ subgroup according to the peripheral white blood cell and platelet count of newly diagnosed patients. The high-risk subgroup of APL patients is usually accompanied by some adverse prognostic factors like FLT3-ITD mutation, non-classical abnormalities of chromosome karyotype, PML/RARa gene complex fusion, more immature and aggressive immunophenotype CD34+/CD56+. Clinically, this subgroup of APL patients is often associated with severe hemorrhagic manifestations, intracranial hemorrhage either at presentation or during the first days of remission induction. By comparison with intermediate-low patients, high-risk patients frequently have increased early mortality and relapse, resulting in poor OS and event-free survival.Citation11–Citation13
Studies have shown that there is an abnormal rise of circulating CD34+ cell level in MDS patients, and have found that the high level of CD34+ cells is a poor biomarker of prognosis. High CD34+ cell count is related to the stratification of disease risk, cytogenetic abnormalities, reduction degree of peripheral blood cell, bone marrow blast count, leukemia-free survival events, and some other prognosis-related factors.Citation14–Citation16
Our published study on APL patients had defined the level of CD34+ cells, namely below 10 × 106/l, and concluded that BM promyelocyte antigen CD34 negative expression might contributed to it. However, previous work only included APL with pancytopenia.Citation10 With more patients enrolled our study, we found some APL patients also had obviously elevated enumeration of CD34+ cells, this study was conducted to comprehensively explore the characteristics of CD34+ cells in APL patients.
Our results demonstrated that some APL patients had significantly elevated CD34+ cell counts. In univariate analysis, a higher CD34+ cell count (≥10 × 106/l) was a biomarker for poor prognosis, because it always associated with some poor clinical indexes and had inferior influence on OS. These indexes included non-classical chromosomal translocation, PML/RARa gene complex fusion, chemotherapy-related APL, WBCs count >10 × 109/l, FLT3-ITD positive mutation, BM promyelocyte CD56/CD34 antigen positive expression and myelofibrosis and marrow necrosis. Except for three patients with higher WBCs count (above 10 × 109/l), no any other APL patient with normal CD34+ cell count had above one index. Although above adverse factors are not independent from WBCs count, a significant correlation between these factors and a worse prognosis has been found in most published studies.Citation11 It is well documented that high level of CD34+ cells indicates inferior prognosis in MDS patients, however, there is a lack of the exact value of CD34+ cells to separate the ‘high’ level from ‘low’ level in APL patients. Knipp et al.Citation17 reported that a cut-off of >10 × 106/l could better distinguish ‘low risk’ MDS patients from ‘high risk’ subjects. Our observation also indicated that a threshold of >10 × 106/l may have the power to distinguish APL patients with adverse clinical prognostic factor from other APL subjects.
Three early death patients had significantly elevated counts of CD34+ cells and correspondingly had more poor indexes (higher WBCs count, PML/RARa gene complex fusion, normal karyotype/complex karyotype abnormalities, CD56/CD34 antigen positive expression, FLT3-ITD positive mutation, and myelofibrosis and marrow necrosis). Since no early death occurred in subgroup with normal CD34+ cell count, the 5-year OS in higher CD34+ cell subgroup was significantly lower than that in normal subgroup. Once complete cytological remission was reached, the absolute counts of CD34+ cells in all patients decreased below 10 × 106/l and remained stable. APL patients with higher CD34+ cell count had usually one or more poor prognostic indexes, life threatening events especially fatal hemorrhage frequently occurred during induction treatment, so the OS rate was obviously inferior to that with normal level CD34+ cell count. Therefore the absolute count of CD34+ cells above 10 × 106/l at initial diagnosis might have indication for early death and could be a candidate biomarker for poor prognosis, clinically intensive transfusion support should be initiated as soon as possible to avoid early death. During following-up, all patients were in complete molecular remission and the level of CD34+ cells was always within 10 × 106/l, so not like other subtype of AL , there was no evidence that elevated count of CD34+ cells may contribute to the progression of APL.
As discussed above, we have showed significant elevation of CD34+ cell number in APL patients and elevated number helps to reliably identify patients with adverse clinical factors and could be a candidate biomarker for worse prognosis. The monitoring of CD34+ cells is practicable, non-invasive and can be easily performed at initial diagnosis and during following-up. Future large-scale trials will determine whether enumeration of CD34+ cells should be included in the work-up of new APL patients.
Disclaimer statements
Contributors Hui Zeng designed this study and collected the cases, Lingdi Yin wrote the article, Qi-Guo Zhang, Ping Li, Yan-hui Yuan, Chao-Yang Guan, Ting Xie checked and revised this paper.
Funding None.
Conflict of interest statement We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of this manuscript.
Ethics approval Ethical approval was not required.
References
- Ahmad EI, Akl HKh, Hashem ME, Elgohary TA. The biological characteristics of adult CD34+ acute promyelocytic leukemia. Med Oncol 2012;29(2):1119–26. doi: 10.1007/s12032-011-9895-y
- Wagner-Souza K, Diamond HR, Ornellas MH, Gomes BE, Almeida-Oliveira A, Abdelhay E, et al. Rhodamine 123 efflux in human subpopulations of hematopoietic stem cells: comparison between bone marrow, umbilical cord blood and mobilized peripheral blood CD34+ cells. Int J Mol Med 2008;22(2):237–42.
- Chicha L, Feki A, Boni A, Irion O, Hovatta O, Jaconi M. Human pluripotent stem cells differentiated in fully defined medium generate hematopoietic CD34– and CD34+ progenitors with distinct characteristics. PLoS One. 2011;6(2):e14733. doi: 10.1371/journal.pone.0014733
- Guan CY, Zhang QG, OuYang J, Zhou RF, Zeng H, Li P, et al. The clinical significance of circulating CD34+ cells in myelodysplastic syndrome and acute leukemia. Acta Universitatis Medicinalis Nanjing (Natural Science) 2010;30(6):847–49.
- Passamonti F, Vanelli L, Malabarba L, Rumi E, Pungolino E, Malcovati L, et al. Clinical utility of the absolute number of circulating CD34-positive cells in patients with chronic myeloproliferative disorders. Haematologica. 2003;88(10):1123–9.
- Alchalby H, Lioznov M, Fritzsche-Friedland U, Badbaran A, Zabelina T, Bacher U, et al. Circulating CD34(+) cells as prognostic and follow-up marker in patients with myelofibrosis undergoing allo-SCT. Bone Marrow Transplant 2012;47(1):143–5. doi: 10.1038/bmt.2011.17
- Zeng H, Zhang QG, OuYang J, Guan CY, Li P, Chen B, et al. The enumeration of circulating CD34+ cells in myeloproliferative neoplasms and its clinical significance. Jiangsu Med J 2014;40(13):1550–2.
- Fuchigami K, Mori H, Matsuo T, Iwanaga M, Nagai K, Kuriyama K, et al. Absolute number of circulating CD34 cells is abnormally low in refractory anemias and extremely high in RAEB and RAEB-t; novel pathologic features of myelodysplastic syndromes identified by highly sensitive flow cytometry. Leuk Res 2000;24(2):163–74. doi: 10.1016/S0145-2126(99)00167-8
- Braulke F, Schanz J, Jung K, Shirneshan K, Schulte K, Schuetze C, et al. FISH analysis of circulating CD34+ cells as a new tool for genetic monitoring in MDS: verification of the method and application to 27 MDS patients. Leuk Res 2010;34(10):1296–301. doi: 10.1016/j.leukres.2010.01.010
- Zeng H, Zhang QG, OuYang J, Xu JY, Li P, Guan CY, et al. Analysis of circulating CD34+ cells in the peripheral blood in pancytopenia. Chin J Clin (Electron Ed) 2013;7(11):5071–3.
- Kelaidi C, Adès L, Fenaux P. Treatment of acute promyelocytic leukemia with high white cell blood counts. Mediterr J Hematol Infect Dis 2011;3(1):e2011038. doi: 10.4084/mjhid.2011.038
- Chillón MC, Santamaría C, García-Sanz R, Balanzategui A, Sarasquete ME, Alcoceba M, et al. Long FLT3 internal tandem duplications and reduced PML/RARa expression at diagnosis characterize a high-risk subgroup of acute promyelocytic leukemia patients. Haematologica 2010;95(5):745–51. doi: 10.3324/haematol.2009.015073
- Xu F, Yin CX, Wang CL, Jiang XJ, Jiang L, Wang ZX, et al. Immunophenotypes and immune markers associated with acute promyelocytic leukemia prognosis. Dis Markers 2014;2014:421906.
- Alhan C, Westers TM, Ossenkopple GJ, van de Loosdrecht AA. Do peripheral blasts count in myelodysplastic syndromes? Leuk Res 2009;33(2):209–11. doi: 10.1016/j.leukres.2008.06.015
- Sullivan SA, Marsden KA, Lowenthal RM, Jupe DM, Jones ME. Circulating CD34+ cells: an adverse prognostic factor in the myelodysplastic syndromes. Am J Hematol 1992;39(2):96–101. doi: 10.1002/ajh.2830390205
- Cesana C, Klersy C, Brando B, Nosari A, Scarpati B, Scampini L, et al. Prognostic value of circulating CD34+ cells in myelodysplastic syndromes. Leuk Res 2008;32(11):1715–23. doi: 10.1016/j.leukres.2008.03.028
- Knipp S, Strupp C, Gatterman N, Hildebrandt B, Schapira M, Giagounidis A, et al. Presence of peripheral blasts in refractory anemia and refractory cytopenia with multilineage dysplasia predicts an unfavourable outcome. Leuk Res 2008;32(1):33–7. doi: 10.1016/j.leukres.2007.02.021