Abstract
Objectives: Human ether-a-go-go-related gene (HERG) K+ channels are shown to be aberrantly expressed in a variety of cancer cells where they play roles in contributing to cancer progression. Myelodysplastic syndromes (MDS) are a group of clinical heterogeneous disorders characterized by bone marrow failure and dysplasia of blood cells. However, the involvement of HERG K+ channels in MDS development is poorly understood.
Methods: The expression of HERG K+ channels in untreated MDS, acute myeloid leukemia (AML) patients and the control group was detected by flow cytometry. The roles of HERG K+ channels in regulation of SKM-1 cell proliferation, apoptosis, and cell cycle were determined by CCK-8 assay and flow cytometry, respectively.
Results: We found that expression of HERG K+ channels in MDS patients was significantly higher than controls and was lower than AML. Percentage of HERG K+ channels on CD34+CD38− cells gradually increased from controls to high-grade MDS subtypes. And HERG K+ channel levels showed an ascending tendency from low-risk to high-risk MDS group. In addition, the CCK-8 assay, apoptosis and cell cycle analysis were performed and showed that blockage of HERG K+ channels decreased the proliferation of MDS cells but rarely had effects on cell apoptosis and cell cycle distribution.
Conclusion: Our study demonstrated that HERG K+ channels might be a potential tumor marker of MDS. These channels were likely to contribute to MDS progression and were helpful for predicting prognosis of MDS. Inhibition of HERG K+ channels might be a novel therapeutic measure for MDS.
Introduction
Human potassium channels are ubiquitously expressed membrane-spanning proteins with versatile functions in cellular physiological processes including maintenance of membrane potential, modulation of cell volume and regulation of cell growth and death.Citation1 Intriguingly, several members among the superfamily of potassium channels appear to be frequently implicated in carcinogenesis, evidence is particularly extensive for the K+ channels encoded by human ether-a-go-go-related gene (herg, Kv11.1, KCNH2).Citation2 HERG K+ channels, one of the evolutionarily conserved families of voltage-activated K+ channels, are known to be constitutively expressed in multiple cell types, such as neurons, cardiomyocytes, and smooth muscle cells, with an essential role in resting potential and action potential repolarization.Citation3,Citation4 However, growing experimental and preclinical data indicate that HERG K+ channels were preferentially expressed in a vast number of human tumor cells of different histogenesis, whereas the corresponding non-cancerous cells have no significant expression of HERG K+ channels.Citation5 In a series of recent studies, HERG K+ channels have been proposed as a biomarker for a wide variety of malignant neoplasms like ovarian cancer, glioblastoma, and leukemia.Citation3 Especially in non-solid carcinomas, HERG K+ channels are reported to contribute to tumor progression by facilitating cancer cell proliferation, migration, neoangiogenesis, and chemotherapy resistance.Citation6–Citation8
Myelodysplastic syndromes (MDS) are defined as a heterogeneous group of clonal hematopoietic stem cell (HSC) malignant disorders, which are characterized by ineffective hematopoiesis resulting in peripheral cytopenias and high risks of evolution (approximately 30–40% of cases) into acute myeloid leukemia (AML).Citation9,Citation10 With the annual incidence exceeding 20 per 100 000 people aged 70 or older, MDS are considered to be one of the most frequent hematologic tumors in patients of aging.Citation11 Up to now, several abnormal molecular events involved in MDS development have been found in MDS patients, such as dysregulation of apoptosis and chemokine signaling pathways, altered expression of genes related to proliferation and differentiation, changes in microRNAs modulating hematopoiesis and defects in ribosomal biogenesis.Citation12,Citation13 In spite of various genomic abnormalities demonstrated so far, the specific pathogenic mechanism of MDS is still poorly understood. Considering the fact that MDS are more difficult to be correctly diagnosed than other hematological neoplasms owing to the pathomorphologic heterogeneity and diversity of clinical courses of this malignancy, it is urgent to investigate reliable and effective molecular biomarkers for MDS.
It is well established that there is a distinct absence of apoptosis and differentiation in the HSCs when MDS progress to AML.Citation9 And as the disease evolves, the percentage of blasts and cytogenetic abnormalities in the bone marrow (BM) increases.Citation9 Despite numerous significant differences reported between MDS and AML, a great deal of overlap in the aberrantly expressed genomic spectrum is present between them, such as MLL, TIM3, and PRDM2.Citation14Citation15–Citation16 In our previous studies, we found the presence of HERG K+ channel expression in AML patients and HERG K+ channels played crucial roles in the pathological processes of leukemia cells, whereas the expression of HERG K+ channels in MDS was unknown.Citation17,Citation18
In this study, we identified the expression signatures of HERG K+ channels in MDS patients and analyzed correlation between expression of HERG K+ channels and clinical parameters of MDS. We found that the percentage of HERG K+ channels in MDS population was obviously higher than controls. An elevated tendency of the level of HERG K+ channels was seen with the increase in malignancy degree of MDS subtypes. In addition, we observed that HERG K+ channel expression in high-risk group was significantly higher than low-risk group. Through blockage of HERG K+ channels, we found that HERG K+ channels played roles in regulation of SKM-1 cell growth. Taken together, our findings indicated that HERG K+ channels had a potential to be a kind of novel biomarker of MDS and these channels might be involved in progression and prognosis stratification of MDS. And HERG K+ channels were possibly to serve as a therapeutic target for the treatment of MDS.
Materials and methods
Sample collection
A total of 36 BM samples from de novo MDS patients (19 males and 17 females, average age 47.4 years (range 10–77)) were obtained in this study. According to the World Health Organization classification, the untreated MDS patients consisted of five subtypes including refractory cytopenia unilineage dysplasia (RCUD), refractory cytopenia with multilineage dysplasia (RCMD), refractory anemia with excess blast I (RAEB-I), and refractory anemia with excess blasts II (RAEB-II). Furthermore, we recruited 20 patients with nonmalignant diseases (8 males and 12 females, mean age 39.5 years (range 10–69)) as the control group. And we collected BM samples from 20 de novo AML patients (14 males and 6 females), with an average age of 46.3 years (ranging from 12 to 69). The clinical characteristics and laboratory parameters of MDS patients and controls were listed in Tables and separately. Specimens of all participants were collected from the Department of Hematology, Union Hospital, Huazhong University of Science and Technology, Wuhan, China. All subjects signed written informed consent before BM puncture and this study was approved by the Institutional Research Ethics Committee of Union Hospital, Huazhong University of Science and Technology.
Table 1 The clinical features and laboratory statistics of de novo MDS patients
Table 2 The clinical characteristics of the control group
Separation of BM mononuclear cells
Mononuclear cells (MNCs) were isolated from BM samples using Ficoll-Histopaque (Sigma-Aldrich, St Louis, USA) by density gradient centrifugation within 24 h after aspiration. Briefly, 2 ml of BM aspirate was diluted up to a volume of 4 ml using phosphate buffer saline (PBS). Diluted BM was layered up the equivoluminal Ficoll-Histopaque, which was then subject to centrifugation at 2000 rpm for 20 min to separate MNC from BM. The MNCs layer was absorbed and washed with 7 ml of PBS and further centrifuged at 1500 rpm for 10 min. Obtained precipitation was submitted to a second centrifugation under the same condition to remove remaining impurities. Subsequently, cell pellets were resuspended in PBS and prepared for flow cytometric analysis.
Flow cytometry
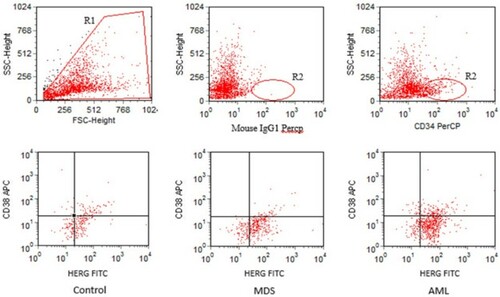
To analyze the expression of HERG K+ channels on cytomembrane, 1 × 106 MNCs in 100 μl of PBS were stained with a solution containing a highly specific rabbit antibody directed against an extracellular epitope of HERG K+ channels (Alomone Labs, Jerusalem, Israel) at 4°C for 20 min. In the meantime, equal MNCs were stained with a solution containing a nonspecific rabbit antibody (Santa Cruz Biotechnologies). After rinsed twice with 2 ml of PBS, cells were incubated for 20 min at 4°C with following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Santa Cruz Biotechnologies), peridinin chlorophyll-protein-labeled anti-CD34 mAb, and allophycocyanin-labeled anti-CD38 mAb (BD Biosciences, CA, USA). Afterwards, MNCs were washed and resuspended in PBS and analyzed on a FCM CaliburTM (BD, Bio) using the CellQuest software (BD, Bio). Appropriate isotype control IgGs were used to eliminate nonspecific signal in the population. First, a total of 30 000 events per tube relative to the whole MNCs were acquired. Second, a minimum of 3000 CD34+ cells were acquired to analyze by means of an electronic gate. Then, we obtained the information of HERG+CD38− compartment among the CD34+ cells.
Cytogenetic analysis
Conventional G-Band karyotype analysis was performed on BM aspirates using established cytogenetic techniques. Typically, BM cells were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 30% fetal bovine serum (Gibco, USA) for 24 h. Colcemid was added to the medium for the last 30 min of culture at a final concentration of 0.05 ug/ml. After collection, cells were treated with the KCl solution (0.068 mol/l) and fixed on slides, then prepared for Giemsa staining. Generally, 20 Giemsa-banded metaphase cells were analyzed per case. Each karyotype was named according to the International System for Human Cytogenetic Nomenclature (ISCN, 2009).
Cell culture and detection of HERG K+ channels
The human MDS cell line SKM-1 was maintained in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (Hyclone, USA) at 37°C in an incubator with 5% CO2 and 95% humidity.
For the analysis of HERG K+ channel expression on the cultured cells, 1 × 106 SKM-1 cells were collected and stained with anti-HERG K+ channel antibody of rabbit source (Alomone Labs, Jerusalem, Israel) for 20 min at 4°C. Then, cells were washed twice with PBS and incubated with FITC-conjugated anti-rabbit IgG (Santa Cruz Biotechnologies) for 20 min at 4°C. After cells were rinsed, cell fluorescence signal was detected by the FCM CaliburTM (BD, Bio) using CellQuest program (BD, Bio).
CCK-8 viability assay
For the investigation of effect produced by E-4031 (Santa Cruz), a specific blocker of HERG K+ channels, on cell proliferation, the SKM-1 cells in logarithmic growth phase were gathered and seeded into 96-well culture plates (Grenier). The cell number was diluted to 20 000/well and cells were incubated with E-4031 at different final concentrations (0, 5, 10, 15, 20, and 30 uM) for 24, 48, or 72 h in 200 μl of culture medium. At the termination of incubation, 20 μl of CCK-8 (Dojindo, Japan) was added to each well and cultivated for another 2 h. Absorbance at 450 nm was read by a Biotek FLx800 microplate reader (Biotek, USA). Each experiment was performed in triplicate.
Cell cycle distribution
The distribution in the cell cycle phases was determined by flow cytometry with propidium iodide (PI) staining. SKM-1 cells were seeded in six-well plates (Grenier) at a density of 5 × 105 cells/well. After treatment with different dosages of E-4031, cells were harvested by centrifugation and washed with PBS, and fixed with ice-cold 75% (v/v) ethanol at 4°C overnight. Then, the cells were washed with PBS to remove fixative and incubated with DNase-free RNase A and PI for 30 min separately in accordance with the manufacturer's instructions (Cell Cycle Detection Kit, KeyGenBioTECH). Stained cells were run through a FCM CaliburTM (BD, Bio) and data were analyzed by ModFit LT program.
Apoptosis assay
Assessment of apoptosis rates was accomplished by Annexin V-PE/7-Amino-Actinomycin (7-AAD) double staining following the protocols (BD Biosciences, CA, USA). SKM-1 cells (5 × 105/well) were treated with E-4031 (0, 10, 30 uM) in the six-well plates for 48–72 h. Afterwards, cells were collected and washed twice with ice-cold PBS, and resuspended in 1 × Binding Buffer at a concentration of 1 × 106 cells/ml. Then, 100 μl of the cell suspension was stained with 5 μl of Annexin V-PE and 5 μl of 7-AAD at room temperature for 15 min in the dark, after which cells were subject to flow cytometric analysis using the CellQuest software (BD Bioscience, CA, USA).
Statistical analysis
The results are expressed as mean value ± standard deviation (SD). Statistical analyses were performed with SPSS software version 21.0. Normality test was performed by the Kolmogorov–Smirnov test, and the Levene test was applied to analyze the homogeneity of variances prior to statistics comparison. For quantitative data, Student's t test and one-way ANOVA test were used to compare differences between two or more groups (for normality data) and the Mann–Whitney U test and Kruskal–Wallis test were applied to analyze non-normality data. Pearson's chi-square test was used to compare groups for qualitative data. In addition, correlation analysis was performed by Pearson and Spearman bivariate correlation analysis. A P-value less than 0.05 was considered statistically significant.
Results
Expression level of HERG K+ channels in each group
With flow cytometry, the expression signature of HERG K+ channels in untreated MDS cohort was analyzed and compared with the AML and control group. We found that the percentage of HERG K+ channels on MNCs in MDS patients was significantly higher than that in controls (41.67 ± 7.07% versus 19.8 ± 2.79%, P < 0.01) and was lower than that in AML patients (41.67 ± 7.07% versus 52.18 ± 9.72%, P < 0.01). Considering that HSCs played important roles in the pathophysiology of MDS and HERG K+ channels might be aberrantly expressed on the MDS HSCs, we compared the amount of HERG K+ channels on CD34+CD38− cells among the three groups (Fig. ). HERG K+ channels on CD34+CD38− cells of MDS patients were associated with a higher percentage when compared with the control group (68.99 ± 7.44% versus 59.44 ± 8.15%, P < 0.01), similarly, the difference of HERG K+ channel level on CD34+CD38− cells between the MDS and AML population was significant (68.99 ± 7.44% versus 74.38 ± 7.95%, P < 0.05).
The percentage of cells expressing HERG K+ channels among the subtypes of MDS cohort
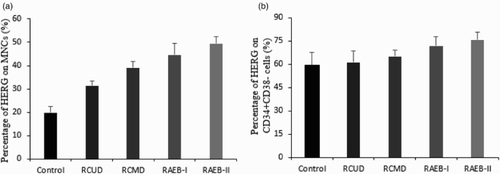
To investigate the association of HERG K+ channels with different MDS subtypes, we analyzed the percentage of HERG K+ channels expression in untreated MDS subtypes included in this study. As was shown in Fig. A, the percentage of HERG K+ channels on MNCs presented an obvious tendency from controls to RAEB-II subtype. The level of HERG K+ channels in each subtype was significantly higher than controls (Control: 19.8 ± 2.79%, RCUD: 31.46 ± 2.08%, RCMD: 39.3 ± 2.63%, RAEB-I: 44.71 ± 4.77%, RAEB-II: 49.4 ± 3.08%, P < 0.01). Meanwhile, we found that profile of HERG K+ channels on CD34+CD38− cells among four subtypes exhibited differences (Fig. B). Expression of HERG K+ channels on CD34+CD38− cells in RAEB-I and RAEB-II was higher than RCMD and RCUD (RCUD: 61.52 ± 7.08%, RCMD: 65.41 ± 3.77%, RAEB-I: 72.02 ± 5.98%, RAEB-II: 75.82 ± 4.78%, P < 0.01), whereas the difference in HERG K+ channel percentage on CD34+CD38− cells among RCMD, RCUD, and controls was not significant (P > 0.05).
Figure 2 HERG K+ channels expression in different MDS subtypes and controls. (A) Positive rate of HERG-expressing cells in MNCs among the MDS subtypes and controls. HERG K+ channels showed an increasing trend from controls to RAEB-II (Control: 19.8 ± 2.79%, RCUD: 31.46 ± 2.08%, RCMD: 39.3 ± 2.63%, RAEB-I: 44.71 ± 4.77%, RAEB-II: 49.4 ± 3.08%, P < 0.01). (B) The percentage of HERG on membrane of CD34+CD38− cells among MDS subtypes and controls. HERG expression in RAEB-I and RAEB-II subgroups was significantly higher than that in RCUD, RCMD, and control group (Control: 59.44 ± 8.15%, RCUD: 61.52 ± 7.08%, RCMD: 65.41 ± 3.77%, RAEB-I: 72.02 ± 5.98%, RAEB-II: 75.82 ± 4.78%, P < 0.01).
Investigation of HERG K+ channel expression and laboratory parameters in de novo MDS patients
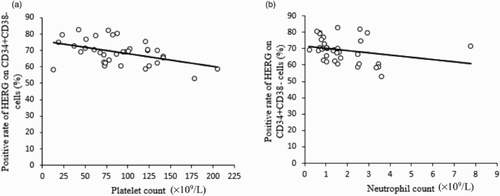
Then, the relationship between the percentage of HERG K+ channels and peripheral blood routine of newly diagnosed MDS patients was explored by correlation analysis. We found that HERG K+ channel amount on CD34+CD38− cells was inversely associated with the neutrophil count (r = −0.397, P < 0.05) and platelet count (r = −0.454, P < 0.01) (Fig. A and B). No significant correlation of HERG K+ channel level with hemoglobulin was observed.
Figure 3 The relationship between percentage of HERG-expressing CD34+CD38− cells and blood routine in MDS patients. (A) The amount of HERG was negatively correlated with platelet count (r = −0.454, P < 0.01). (B) HERG expression was reversely correlated with neutrophil count (r = −0.397, P < 0.05).
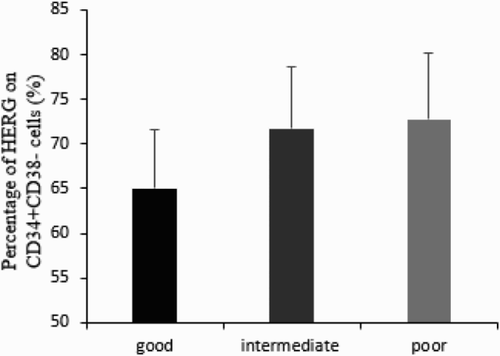
In addition, we analyzed the difference of level of HERG K+ channels in disparate karyotype condition, which were divided into three groups including good (normal, -Y, del (5q), del (20q)), intermediate (other cytogenetic abnormalities), and poor group (more than three variations or chromosome 7 abnormalities) and detected differences among the three groups (good: 65.05 ± 6.51%, intermediate: 71.83 ± 6.73%, and poor: 72.84 ± 7.29%, P < 0.05) (Fig. ).
Analysis of HERG K+ channels and prognosis classification
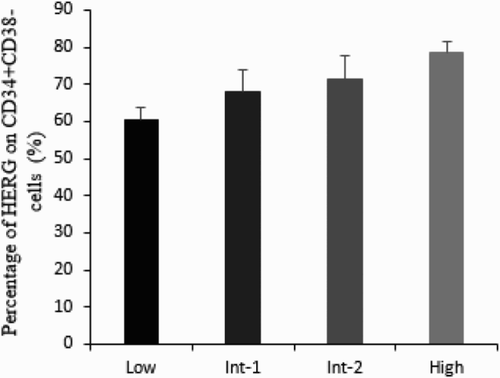
The prognosis stratification of MDS patients was performed according to the International Prognostic Scoring System (IPSS) scores and the untreated MDS cohort was categorized into four risk groups: Low-R (0, n = 6), Int-1-R (0.5∼1.0, n = 14), Int-2-R (1.5∼2.0, n = 12), and High-R (≥2.5, n = 4). As was shown in Fig. , the level of HERG K+ channels on CD34+CD38− cells presented an increasing tendency from low-risk to high-risk MDS group (Low-R: 60.21 ± 4.74%, Int-1-R: 68.05 ± 7.09%, Int-2-R: 71.31 ± 4.75%, and High-R: 78.46 ± 2.9%, P < 0.01).
Figure 5 The level of HERG on CD34+CD38− cells in different prognosis groups. With the risk elevated, the expression of HERG increased gradually. There was a significantly positive correlation between the percentage of HERG and risk stratification (Low-R: 60.21 ± 4.74%, Int-1-R: 68.05 ± 7.09%, Int-2-R: 71.31 ± 4.75%, High-R: 78.46 ± 2.9%, P < 0.01).
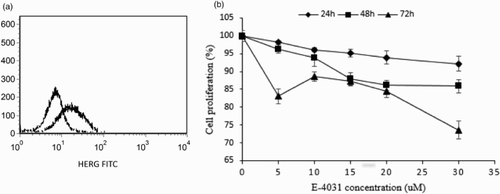
Effects of E-4031 on the proliferation, apoptosis, and cell cycle of SKM-1 cells
With the flow cytometric method, HERG K+ channels were observed to be highly expressed on the membrane of SKM-1 cells (Fig. A). Then, to test whether HERG K+ channel activity was involved in proliferation of MDS cells, we analyzed the effect of HERG K+ channels blockage on SKM-1 cells (Fig. B). The CCK-8 assay demonstrated that E-4031 suppressed the growth of SKM-1 cells in a dose-dependent manner. There was a significant declining tendency in the number of viable cells with the increasing E-4031 concentration. Furthermore, the inhibition rate of growth increased with the prolongation of incubation time at the same concentration of E-4031. And the percentages of SKM-1 cells were 92.13 ± 2.1, 85.89 ± 1.84, and 73.57 ± 2.53% at 24, 48, and 72 h, respectively, exposed to 30 uM E-4031 (P < 0.01), suggesting that the inhibitory effect of HERG K+ channel blockage on cell proliferation was time dependent. Whereas, we found that no increase in the rate of apoptotic cells was observed after treatment of cells with E-4031 and no significant changes in the distribution of cell cycle phase were detected among groups treated with different concentrations of E-4031 (data were not shown). Furthermore, we found that expression of HERG K+ channels on SKM-1 cells was not inhibited by 20 μM E-4031 (0 h: 58.83 ± 2.02%; 24 h: 61.55 ± 3.79%; 56.06 ± 3.91%, P > 0.05).
Figure 6 The effects of HERG blockage on biological activities of SKM-1 cells. (A) The HERG K+ channels were highly expressed on SKM-1 cells. The line on the left represented the isotype control and the line on the right represented HERG-bearing SKM-1 cells. (B) Time-dependent and dose-dependent effects of E-4031 on the proliferation of SKM-1 cells. Cells were treated with different dosages of E-4031 for 24, 48 and 72 h. Cells without treatment were used as control, with proliferation set at 100%. Each data point represented the mean ± SD of three independent experiments.
Discussion
Ion channels constitute a novel area of research in oncology, especially for HERG K+ channels, which have been reported to emerge as pivotal players in tumor-related processes.Citation6,Citation7,Citation19 It is well known that HERG K+ channels are associated with the progression of a variety of malignant neoplasms, such as colorectal cancer and leukemia.Citation7,Citation20 However, the involvement of HERG K+ channels in MDS has not been systematically investigated. In this study, we presented evidence that HERG K+ channels were aberrantly over-expressed on CD34+CD38− cells in MDS patients with respect to patients with nonmalignant diseases. Clinicopathological analysis revealed that the percentage of HERG K+ channels on CD34+CD38− cells among untreated MDS tended to elevate with the increase in prognosis risk. In addition, selective blockade of HERG K+ channels had antiproliferative effects on MDS cells.
Accumulating lines of evidence demonstrate that HERG K+ channels could serve as a reliable bioactive marker of several types of cancer.Citation2,Citation20,Citation21 Our findings indicated that the level of HERG K+ channels on the MNCs in every subtypes of de novo MDS cohort was significantly higher than the controls. In addition, HERG K+ channels expression on CD34+CD38− cells showed an ascending trend from controls to RAEB-II MDS subtype. These findings indicated that HERG K+ channels were abnormally over-expressed in MDS patients, which suggested that HERG K+ channels had a potential to be a tumor marker of MDS. However, this viewpoint was needed to be further verified through enlarging samples.
Cancer stem cells were described as a subgroup of rare cells with unlimited potential for self-renewal and extensively proliferation leading to carcinogenesis.Citation22 There was evidence in the literature showing that cancer stem cells were essential for the occurrence and development of hematological malignancies.Citation22,Citation23 As normal HSCs and tumor stem cells were both contained with the CD34+CD38− population,Citation23 we analyzed the expression of HERG K+ channels on CD34+CD38− cells in MDS patients. Our results presented that levels of HERG K+ channels on CD34+CD38− population among MDS subtypes were different and an elevated tendency in the percentage of HERG K+ channels was observed from RCUD to RAEB-II subgroup. It was indicated that decrease in blood cells and abnormality of karyotype could, to some degree, reflect the level of malignancy of MDS. Data reported in the paper showed that amount of HERG K+ channels on CD34+CD38− subset was obviously negatively correlated with platelet and neutrophil counts and karyotype poor group showed high expression of HERG K+ channels. These results, together with the fact that MDS malignancy degree tended to increase from RCUD to RAEB-II,Citation24 indicated that HERG K+ channels expression on CD34+CD38− cells might be positively associated with the malignancy degree of MDS. In addition, given that abnormalities of bioactive molecules in HSCs were closely related to pathogenesis of MDS and HERG K+ channels distributed on HSCs membrane had been proved to be implicated in the progression of myeloid neoplasms,Citation6,Citation13 aberrant over-expression of HERG K+ channels on CD34+CD38− cells in MDS patients was likely to be correlated with the oncogenesis of MDS.
It was noteworthy that expression of HERG K+ channels exhibited relatively high level on CD34+CD38− cell membrane in control population. This phenomenon discovered was possibly attributed to the physiological mechanisms that HERG K+ channels were mainly expressed in progenitors and stem cells, which began to divide or grow in response to cytokine stimulation.Citation5,Citation6
HERG K+ channels had been proposed as a molecular marker associated with recurrence and invasive phenotype of tumors.Citation7,Citation20 In accordance with the IPSS scores, newly diagnosed MDS patients were classified as subgroups with four different clinical outcomes. We observed that the higher the HERG K+ channels expressed, the greater the prognosis risk was. These findings, together with the previous studies that up-regulation of HERG K+ channels was related to early relapse and short overall survival of cancers,Citation7,Citation21 suggested that HERG K+ channels could act as an effective prognostic indicator for MDS. However, considering that cases included in this study were not numerous and lacking follow-up of MDS patients in remission or recurrence, efficacy of HERG K+ channels for predicting the outcomes of MDS needed to be further confirmed.
In order to further explore the involvement of HERG K+ channels in pathogenic mechanisms underlying MDS, we analyzed the potential functions of HERG K+ channels in biological processes of SKM-1 cells. We found that inhibition of HERG K+ channels markedly decreased cellular proliferation but produced rarely effects on cell cycle and apoptosis. Consistent with other studies, HERG K+ channels had already been shown to be necessary for growth of cancer cells through specific activities independent of cell cycle such as interaction with TNFR1 protein, which could activate NF-κB to facilitate cell proliferation and favor transduction of growth signals by the MAP kinase/c-fos pathway.Citation25,Citation26 Furthermore, previous studies indicated that E-4031 was not involved in the growth of cells, which did not express HERG K+ channels.Citation6,Citation26,Citation27 These findings demonstrated that physiological activity of HERG K+ channels was crucial for MDS cell proliferation and reminded us that pharmacological blockage of HERG K+ channels was promising to be a therapeutic method for MDS. However, we were informed that HERG K+ channels were normally expressed on cardiomyocytes and blockade of HERG K+ channels could cause long QT syndromes with an increased risk of lethal torsade de pointes arrhythmia,Citation4,Citation28 so we should focus on researching channel inhibitors specially targeting cancer cells, which expressed HERG K+ channels in the near future.
Conclusion
On the whole, this was the first study showing that HERG K+ channels were abnormally highly expressed in MDS patients and these channels had potentials to act as a biomarker of MDS. Moreover, HERG K+ channels were possibly involved in pathophysiological processes of MDS. We proposed that expression level of HERG K+ channels could be utilized for the stratification of prognosis among MDS patients. In addition, there was a possibility for HERG K+ channels to be applied as a novel target for MDS therapy.
Disclaimer statements
Contributors L.L. performed all experiments and drafted the manuscript. W.D. and W.L. helped to analyze the data. D.G. contributed scientific support. H.L. and X.H. designed and supervised the study. All authors read and approved the final manuscript.
Funding This study was supported by the National Natural Science Foundation of China (No. 81170462).
Conflict of interest statement The authors have stated that they have no conflicts of interest.
Ethics approval This study was approved by the Institutional Research Ethics Committee of Union Hospital, Huazhong University of Science and Technology.
References
- Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev. 2000;52:557–94.
- Jehle J, Schweizer PA, Katus HA, Thomas D. Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis. 2001;2:e193. doi: 10.1038/cddis.2011.77
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–478. doi: 10.1152/physrev.00036.2011
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–9. doi: 10.1038/nature04710
- Wang Z. Roles of K+ channels in regulating tumor cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–86. doi: 10.1007/s00424-004-1258-5
- Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–8. doi: 10.1038/sj.leu.2402572
- Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V, et al. VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood. 2007;110:1238–50. doi: 10.1182/blood-2006-02-003772
- Pillozzi S, Masselli M, De Lorenzo E, Accordi B, Cilia E, Crociani O, et al. Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood. 2011;117:902–14. doi: 10.1182/blood-2010-01-262691
- Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–29. doi: 10.1038/nrc2047
- Pellagatti A, Boultwood J. The molecular pathogenesis of the myelodysplastic syndromes. Eur J Haematol. 2015;95:3–15.
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. doi: 10.1056/NEJMra0902908
- Pellagatti A, Cazzola M, Giagounidis A, Perry J, Malcovati L, Della Porta MG, et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia. 2010;24:756–64. doi: 10.1038/leu.2010.31
- Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012;12:849–59. doi: 10.1038/nrc3321
- Tang G, DiNardo C, Zhang L, Ravandi F, Khoury JD, Huh YO, et al. MLL gene amplification in acute myeloid leukemia and myelodysplastic syndromes is associated with characteristic clinicopathological findings and TP53 gene mutation. Hum Pathol. 2015;46:65–73. doi: 10.1016/j.humpath.2014.09.008
- Tao JL, Li LJ, Fu R, Wang HQ, Jiang HJ, Yue LZ, et al. Elevated TIM3+ hematopoietic stem cells in untreated myelodysplastic syndromes displayed aberrant differentiation, overproliferation and decreased apoptosis. Leuk Res. 2014;38:714–21. doi: 10.1016/j.leukres.2014.03.018
- Xie W, Li X, Chen X, Huang S, Huang S. Decreased expression of PRDM2 (RIZ1) and its correlation with risk stratification in patients with myelodysplastic syndrome. Br J Haematol. 2010;150:242–4.
- Li H, Liu L, Guo L, Zhang J, Du W, Li X, et al. HERG K+ channel expression in CD34+/CD38-/CD123(high) cells and primary leukemia cells and analysis of its regulation in leukemia cells. Int J Hematol. 2008;87:387–92. doi: 10.1007/s12185-008-0056-9
- Zheng F, Li J, Du W, Wang N, Li H, Huang S. Human ether-a-go-go-related gen K+ channels regulate shedding of leukemia cell-derived microvesicles. Leuk Lymphoma. 2012;53:1592–8. doi: 10.3109/10428194.2012.661855
- Li H, Du YM, Guo L, Jie S, Zhang S, Du W, et al. The role of hERG1 K+ channels and a functional link between hERG1 K+ channels and SDF-1 in acute leukemic cell migration. Exp Cell Res. 2009;315:2256–64. doi: 10.1016/j.yexcr.2009.04.017
- Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–11. doi: 10.1158/0008-5472.CAN-03-2360
- Ding XW, Luo HS, Luo B, Xu DQ, Gao S. Overexpression of hERG1 in resected esophageal squamous cell carcinomas: a marker for poor prognosis. J Surg Oncol. 2008;97:57–62. doi: 10.1002/jso.20891
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730
- Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. Hematol Am Soc Hematol Educ Program. 2002:136–61.
- Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–8.
- Afrasiabi E, Hietamäki M, Viitanen T, Sukumaran P, Bergelin N, Törnquist K. Expression and significance of HERG (KCNH2) potassium channels in the regulation of MDA-MB-435S melanoma cell proliferation and migration. Cell Signal. 2010;22:57–64. doi: 10.1016/j.cellsig.2009.09.010
- Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M, et al. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem. 2003;278:2947–55. doi: 10.1074/jbc.M210789200
- Patanѐ S. HERG-targeted therapy in both cancer and cardiovascular system with cardiovascular drugs. Int J Cardiol. 2014;176:1082–5. doi: 10.1016/j.ijcard.2014.07.129