Abstract
Introduction: We report a chronic persistent Parvovirus B19 (PVB19) infection despite long-term immunoglobulin substitution intravenous immunoglobulin (IVIG) and tapering of immune-suppressive therapy in a 41-year-old patient after allogeneic haematopoietic stem cell transplantation (alloHSCT) and long-term immune-suppressive therapy due to a steroid-refractory graft versus host disease (GvHD).
Clinical course: More than 18 month after alloHSCT the patient acquired a de novo transfusion-dependent pure red cell aplasia (PRCA) due to a PVB19 infection. Despite prompt tapering of GvHD-directed therapy and application of various IVIG regimens, transfusion-dependent anaemia (fourerythrocyte concentrates a month) persisted, and a high PVB19 replication is still evident for more than 3.5 years. Virological analysis at different time points showed a very high PVB19 load in the blood (range: 6.79E9–1.56E11), as well as highly elevated PVB19-IgG (range: 1.95–3.34) and -IgM (range: 1.97–9.74) levels in serology testing. Other virological parameters were not significantly elevated. After 30 months, a bone marrow (BM) examination still revealed a highly dysplastic erythropoiesis without any cellular maturation, and a high-grade expression of PVB19 within the dysplastic erythropoietic progenitor cells, consistent with a PRCA due to a PVB19 infection of the BM. We suggest that PRCA was most probably caused by a primary PVB19 infection of unknown source following alloHSCT with a PVB19-negative donor.
Conclusion: PRCA due a PVB19 infection of the BM may persist over a long-time, despite prolonged administration of various IVIG regimen and tapering of GvHD-directed therapy. The case emphasizes the importance of PVB19 monitoring in heavily pre-treated haematological patients. Currently, PVB19-directed treatment options are extremely limited and optimized therapeutic strategies are urgently needed.
Introduction
Parvovirus B19 (PVB19) is a single-stranded DNA virus with a high tropism to erythroid progenitor cells.Citation1 PVB19 infection is usually self-limiting in immunocompetent patients, and viraemia is cleared within a few weeks.Citation2,Citation3 In immunodeficient patients PVB19 viraemia may persist, and among others patients may develop a pure red cell aplasia (PRCA).Citation2,Citation4,Citation5 In these patients, intravenous immunoglobulin (IVIG) therapy effectively corrected haemoglobin levels and PVB19 PCR results became negative within a few months in most patients.Citation6 Here, we report a case of a chronic persistent PVB19 bone marrow (BM) infection despite long-term IVIG therapy in a 41-year-old multiple myeloma patient after allogeneic haematopoietic stem cell transplantation (alloHSCT).
Material and methods
PVB19 antibody detection in patient's sera was performed by enzyme immunoassay using the Enzygnost® kit (Siemens, Marburg, Germany) according to manufacturers instructions. A lineblot system (recomline®, Mikrogen, Neuried, Germany) was used to determine IgG and IgM antibody profiles and avidity. The concentration of PVB19 in plasma and BM was measured by PCR (cobas®, Roche, Basel, Switzerland). Immunohistochemistry staining was conducted with antiPVB19 antibody (Dako, Glostrup, Denmark). Light microcopy was performed using AxioLab & AxioCam® (Zeiss, Jena, Germany). Polyvalent EBV IgG analysis was done using Enzygnost® (Siemens, Marburg, Germany). EBV-IgM line blot analysis was performed with recomline® (Mikrogen, Neuried, Germany) and heterophile antibody detection with Paul Bunnell test (biokit, Barcelona, Spain). Quantitative EBV PCR was performed using LightCycler® (Roche, Basel, Switzerland). Flow cytometry was performed with Canto II (BD Biosciences).
IVIG therapy included three different preparations according to availability: Octagam® (Octapharma GmbH, Langenfeld, Germany), Privigen® (Tisida GmbH, München, Germany), and Gamunex® (Grifolis Deutschland GmbH, Frankfurt a.M., Germany).
Case report
AlloHSCT was performed in September 2009 due to a refractory IgD-lambda multiple myeloma stadium IIIB (Salmon/Durie) with a history of an osteolytic fraction, acute renal failure, and large extra-medullary manifestations. Within 2 years after primary diagnosis, therapy included two cycles of high-dose dexamethasone, 2 × 3 cycles of a bortezomib-based chemotherapy, three cycles of a lenalidomid-based chemotherapy, neurochirurgic surgery (laminectomy), interventional radiation of the spine and extra-medullary manifestations, and two autologous stem cell transplantations after myeloablative therapy with melphalan. After reduced intensity conditioning with treosulfan (3 × 14 g m−2), fludarabine (5 × 30 mg m−2), and anti-thymocyte globulin (ATG Freseinus® 60 mg m−2), the patient received a strong allograft of 8.5 × 106 CD34-positive peripheral blood stem cells per kg bodyweight from an unrelated donor (human lenkocyte antigen match 8/10, mismatches at B-antigen and C-allele loci; no AB0-incompatibility). Subject had a timely haematopoietic engraftment with normal blood cell counts within 30 days after transplantation and achieved a complete remission of multiple myeloma as demonstrated by a repeatedly negative immunofixation electrophoresis. Soon after engraftment, the patient developed an acute 2-organ grade III GvHD (muco-cutaneous and intestinal) despite the prophylactic treatment with ciclosporin (CSA) and mycophenolate mofetil (MMF). Intestinal GvHD was initially refractory to steroid therapy, but could be controlled by a prolonged triple therapy including steroids, the TNFα inhibitor infliximab, and tacrolimus. Muco-cutaneous GvHD showed an initial response, but turned into chronic stage requiring a permanent immunosuppression with steroids and MMF. A GvHD-directed therapy with the mTOR inhibitor everolimus was less effective and had to be replaced due to a transfusion-dependent thrombotic microangiopathy that was completely reversible after returning to a MMF-based therapy.
More than 18 months after alloHSCT the patient acquired a de novo transfusion-dependent anaemia (Hb range 4.6–5.3 mmol/l). At onset of anaemia, the patient displayed a mild chronic GvHD of the skin according to NIH consensus, and received an immunosuppressive medication with MMF (2 × 360 mg daily) and prednisolone (5 mg per day). While white blood cell count and platelets remained unaffected, anaemia was accompanied by an abrupt drop of reticulocyte count (Fig. ), and was diagnosed as PRCA due to a PVB19 infection. Other reasons could be excluded. Therapy of PRCA included a gradual tapering of chronic GvHD-directed therapy and intravenous administration of polyvalent immunoglobulins. IVIG therapy was started with 0.4 g/kg bodyweight for five consecutive days and was repeated every 4 weeks. After five cycles, IVIG administration was changed to single doses of 20–30 g per day every 3–4 weeks with respect to the ambulant setting. Despite the long-term application of IVIG therapy and final cessation of GvHD-directed therapy, transfusion-dependent anaemia persisted with an unchanged need of 4–5 to erythrocyte concentrates every month. High PVB19 replication is still evident until this present day. Thirty months after onset of PRCA, a BM examination still revealed a high-grade dysplastic erythropoesis without recognizable maturation, and persistent expression of PVB19 within the erythropoietic progenitor cells, consistent with a chronic PVB19 infection of the BM (Fig. A–C). Plasma cells reached an interstitial infiltration of 15% of all core cells, but demonstrated a polyclonal distribution without any evidence of relapse of multiple myeloma (no plasma cell stained positive for IgD-lambda). Complete remission of multiple myeloma was further confirmed by a negative immunofixation electrophoresis and normal serum free light chain levels.
Figure 1 (A) Dysplastic BM erythropoiesis; intranuclear inclusion giant pronormoblasts (Giemsa). (B) Immunhistochemical contrasting of erythropoiesis: abnormal BM dysplasia, no aplasia. (C) Immunohistochemical detection of PVB19-positive cells in BM (antiPVB19 Ab)
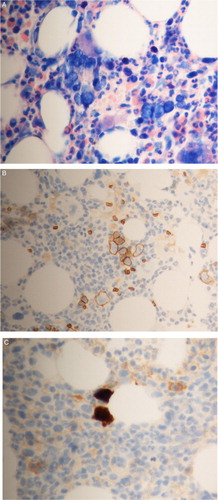
Figure 2 Correlation of PVB19 diagnostic parameters (PVB19 viral load, lgG/lgM immunoblot) with anemia laboratory values (reticulocytes, hemoglobin) over time during treatment (prednisolone, MMF, IVIG)
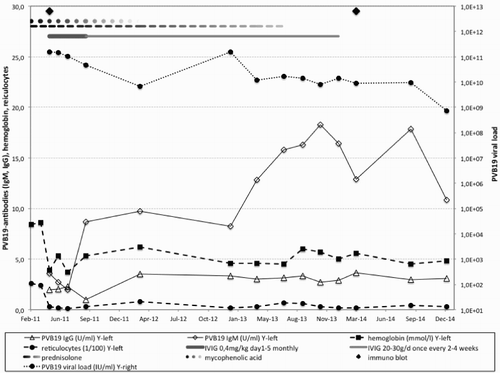
Virological analysis at different time points showed a very high PVB19 load in the blood (6.79×109–1.56×1011 IU/ml) as well as highly elevated PVB19-IgG log10 titre (1.95–3.34, normal range < 0.9) and -IgM log10 titer (1.97–18.30, normal range < 0.9) in serology testing (Fig. ). IgG-immunoblot in May 2011 revealed antibodies with intermediate avidity and epitope pattern compatible with recent infection. IgM-immunoblot was not performed. An immunoblot performed in March 2014 showed a matured epitope pattern with remaining early bands (Fig. ). Avidity could not be determined due to a lack of determining bands. The IgM pattern at this time point remained immature. However, at this late state interpretation of serologic results is hampered by previous IVIG administration.
Figure 3 PVB19 immunoblot (recomline®): line 1: PVB19-IgG-immunoblot (positive): Vp-2p +++, VP-1S +, VP-2r [+]; line 2: PVB19-IgG avidity: (VP-1S); line 3: PVB19 IgM-immunoblot (positive): Vp-2p +++, VP-2r +, Vp-C [+]. Abbreviations: Vp-2p (main capsid antigen- conformation epitope), VP-1S (VP1-unique region), VP-2r (main capsid antigen- linear epitope), Vp-C (C – terminal conjoint half of structure proteins VP1 and VP2).
![Figure 3 PVB19 immunoblot (recomline®): line 1: PVB19-IgG-immunoblot (positive): Vp-2p +++, VP-1S +, VP-2r [+]; line 2: PVB19-IgG avidity: (VP-1S); line 3: PVB19 IgM-immunoblot (positive): Vp-2p +++, VP-2r +, Vp-C [+]. Abbreviations: Vp-2p (main capsid antigen- conformation epitope), VP-1S (VP1-unique region), VP-2r (main capsid antigen- linear epitope), Vp-C (C – terminal conjoint half of structure proteins VP1 and VP2).](/cms/asset/dda858b3-1eaf-4614-bf98-7a40e8da13cc/yhem_a_1183288_f0003_c.jpg)
During further virology check-up EBV IgG was positive and EBV IgA negative (ELISA). EBV IgG line blot showed the pattern of a past infection (bands for EBNA, VCA, and IEA) with no signs of reactivation (EBV IgA line blot remained negative). However, EBV qPCR was positive with a viral load ranging between 5.0E3 and 1.29E6 IU/ml at different time points measured. Testing for other viruses (CMV, HSV, VZV, FSME) was negative.
In flow cytometry, the absolute amount of CD4+ cells was reduced (137 cells/µl; range: 441–2156 cells/µl). CD8 + cells were within normal range (716 cells/µl; range: 125–1312 cells/µl), thus resulting in a significantly reduced CD4/CD8 ratio (0.19; range: 0.9–3.6). Absolute numbers of CD19 B cells were slightly reduced (95 cells/µl; range: 107–698 cells/µl) and NK cells were highly elevated (1355 cells/µl; range: 95–640 cells/µl). At first diagnosis of PVB19 infection, serum levels of IgA (0.60 g/l) and IgM (0.65 g/l) were clearly suppressed. During the most recent analysis, immunglobulin G level was highly elevated (30.90 g/l; range 7.51–15.60 g/l). Serum IgG immunoglobulins were subclassified for IgG1 (15.50 g/l; range 2.8–8.0 g/l), IgG2 (3.32 g/l; range: 1.15–5.70 g/l), IgG3 (1.74 g/l; range: 0.24–1.25 g/l) and IgG4 (0.128 g/l; range: 0.052–1.250 g/l). Ferritin level was highly elevated (5520.6 µg/l; range: 22–322 µg/l).
Discussion
Here we reported a case of a young multiple myeloma patient who developed a chronic persistent PRCA due to a PVB19 infection 18 month after alloHSCT. The origin of PVB19 infection remains unknown. Blood products may contain PVB19, and a transmission by blood products is possible.Citation2,Citation7 But this is unlikely in the reported case, since the last transfusion was administered more than 4 month before onset of PRCA. A primary infection via the respiratory route appears most likely, even though the patient demonstrated no typical clinical features like fever or exanthema. For the majority of immunodeficient patients clinical manifestations are lacking, and anaemia may represent the first and only sign of infection.Citation6 However, a reactivation cannot finally be excluded. Cassinotti et al. demonstrated that PVB19 may persist in the BM of asymptomatic individuals.Citation8 In our case, the patient received a graft from a PVB19-negative donor, and obtained an intensified immunosuppressive therapy after development of GvHD.
In immunocompetent individuals, viraemia is usually cleared within a few weeks by the production of neutralizing antibodies against the capsid proteins VP1 and VP2.Citation2 While the early IgM antibodies are mainly directed VP2-specific epitopes, a life-long protection against reinfection relies on the later production of IgG antibodies directed against the VP1-unique region.Citation9 In case of a persistent viraemia, patients may develop IgG antibodies directed against the non-structural protein NS1, which usually demonstrate only weak neutralizing activity.Citation9,Citation10
At primary diagnosis of PVB19 infection in May 2011, the patient exhibited a very high viral load of >1×1011 IU/ml, and serologic testing revealed a significant elevation of PVB19-specific IgM (3.58 U/ml; norm < 0.9) as well as IgG (1.95 U/ml; norm < 0.9) antibodies consistent with a recent infection. Follow-up testing revealed a continuously high viral load and an even further increase of PVB19-directed IgM antibodies in the following years (Fig. ). In a 2nd immunoblot performed almost 3 years after primary diagnosis the IgM pattern remained immature. The IgG-blot also demonstrated remaining early bands and only a weak band of neutralizing VP1 antibodies (Fig. ). In the absence of neutralizing antibodies, the constant elevation of PVB19-directed IgM antibodies results most likely from the continuous viral stimulation.
Persistence of PVB19 in immunocompromised patients is mainly caused by an impairment of B-cell function.Citation11 A defect in antibody maturation and an inability to produce sufficient amounts of neutralizing antibodies is obvious in this heavily pre-treated patient after alloHSCT and intensified GvHD-directed therapy. Even after cessation of immunosuppressive therapy, the number of peripheral B-cells remained below the normal range and CD4/CD8 ratio was still significantly reduced. The increased BM infiltration with polyclonal plasma cells and the significantly elevated IgG serum levels might be interpreted as signs of a continuously activated but insufficient immune response.
Interestingly, despite PVB19 persistence for more than 3 years no NS1-directed antibodies could be found. The significance of NS1-directed antibodies is still ambiguous. As NS1 antibodies are preferentially found in patients with a chronic viraemia and involvement of synovial or myocardial infection, these antibodies may indicate an infection of cells outside the erythroid lineage.Citation2 In our patient, the continuous involvement of the erythoid lineage is clearly documented by the immunhistochemical detection of PVB19-positive erythropoietic progenitor cells (Fig. C). In the absence of a sufficient amount of neutralizing antibodies, these PVB19-positive progenitor cells may represent a source of continuous reinfection.
Therapeutic options for patients with PRCA due to a chronic persistent PVB19 infection are limited and include a reduction of immunosuppressive therapyCitation3,Citation12,Citation13 and/or the application of high-dose IVIGs.Citation5,Citation7,Citation14 Since a majority of general population is seropositive for a past PVB19 infection, most IVIG formulations contain considerable amounts of neutralizing PVB19-directed antibodies, even though the exact content of PVB19-specific antibodies is not always known. In a retrospective analysis of 10 own patients and 123 patients from the literature, Crabol et al.Citation6 reported a correction of haemoglobin levels after the first IVIG course in over 90% of patients with a relapse of about 30% within a mean of 4 month. In our case, administration of five courses of high-dose IVIG therapy was less effective and had no influence of viraemia, haemoglobin levels, and reticulocyte count (Fig. ).
Owing to a lack of prospective data, the optimal schedule of IVIG treatment remains unknown. Doses of 0.4 mg/kg for 5 days or 1 mg/kg for 2–3 days have been commonly proposed, while treatment with low or intermediate doses has often failed.Citation2,Citation6,Citation14 Even less is known, about the optimal timing and duration of IVIG courses. After early cessation of therapy, a relapse of PCRA is likely.Citation6 Otherwise, patients who demonstrated little response during the first courses of IVIG administration may profit from a consequent, long-time continuation of IVIG therapy.Citation7,Citation15 In our case, a continuation of IVIG therapy with 0.3–0.5 g/kg (20–30 g absolute) every 3–4 weeks remained ineffective. Presumably, higher doses are also needed for long-term therapy.
Currently, the patient still suffers from PRCA with an unchanged need of approximately 4–5 erythrocyte concentrates every month. Despite continuous administration of chelation therapy with deferasirox, iron overload as reflected by highly elevated ferritin levels becomes a growing problem. To date, no PVB19-directed drugs are available for additional use in our patient.
Conclusion
This case shows the importance of PVB19 monitoring in heavily pre-treated haematological patients after alloHSCT. In contrast to previous reports of improvement of PVB19 infection after IVIG therapy, this treatment failed in our patient. We therefore want to stress the point that PVB-directed treatment options are currently limited and optimized therapeutic strategies in PVB19 infections are urgently needed.
Disclaimer statement
Contributors All authors reviewed manuscript.
Funding None.
Conflicts of interest No conflicts-of-interest.
Ethics approval No EC required.
ORCID
Iver Petersen http://orcid.org/0000-0002-3535-3098
References
- Bultmann BD, Klingel K, Sotlar K, Bock CT, Kandolf R. Parvovirus B19: a pathogen responsible for more than hematologic disorders. Virchows Arch. 2003;442(1):8–17.
- Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15(3):485–505. doi: 10.1128/CMR.15.3.485-505.2002
- Watanabe D, Taniguchi T, Otani N, Tominari S, Nishida Y, Uehira T, et al. Immune reconstitution to parvovirus B19 and resolution of anemia in a patient treated with highly active antiretroviral therapy. J Infect Chemother. 2011;17(2):283–7. doi: 10.1007/s10156-010-0111-3
- Breinholt JP, Moulik M, Dreyer WJ, Denfield SW, Kim JJ, Jefferies JL, et al. Viral epidemiologic shift in inflammatory heart disease: the increasing involvement of parvovirus B19 in the myocardium of pediatric cardiac transplant patients. J Heart Lung Transplant. 2010;29(7):739–46. doi: 10.1016/j.healun.2010.03.003
- Pinto V, Grandy J, Zambrano P, Corta B, Salas P, Salgado I, et al. Severe anemia from parvovirus b19 infection in pediatric renal transplant recipients: two case reports. Transplant Proc. 2008;40(9):3261–4. doi: 10.1016/j.transproceed.2008.03.127
- Crabol Y, Terrier B, Rozenberg F, Pestre V, Legendre C, Hermine O, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis. 2013;56(7):968–77. doi: 10.1093/cid/cis1046
- Plentz A, Hahn J, Holler E, Jilg W, Modrow S. Long-term parvovirus B19 viraemia associated with pure red cell aplasia after allogeneic bone marrow transplantation. J Clin Virol. 2004;31(1):16–9. doi: 10.1016/j.jcv.2004.05.015
- Cassinotti P, Burtonboy G, Fopp M, Siegl G. Evidence for persistence of human parvovirus B19 DNA in bone marrow. J Med Virol. 1997;53(3):229–32. doi: 10.1002/(SICI)1096-9071(199711)53:3<229::AID-JMV8>3.0.CO;2-A
- Modrow S, Dorsch S. Antibody responses in parvovirus B19 infected patients. Pathol Biol (Paris). 2002;50(5):326–31. doi: 10.1016/S0369-8114(02)00302-4
- Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young NS, et al. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol. 1999;73(3):1974–9.
- Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350(6):586–97. doi: 10.1056/NEJMra030840
- Mylonakis E, Dickinson BP, Mileno MD, Flanigan T, Schiffman FJ, Mega A, et al. Persistent parvovirus B19 related anemia of seven years’ duration in an HIV-infected patient: complete remission associated with highly active antiretroviral therapy. Am J Hematol. 1999;60(2):164–6. doi: 10.1002/(SICI)1096-8652(199902)60:2<164::AID-AJH16>3.0.CO;2-4
- Shimmura H, Tanabe K, Ishikawa N, Tokumoto T, Toda F, Toma H. Discontinuation of immunosuppressive antimetabolite for parvovirus B19-associated anemia in kidney transplant patients. Transplant Proc. 2000;32(7):1967–70. doi: 10.1016/S0041-1345(00)01515-3
- Liang TB, Li DL, Yu J, Bai XL, Liang L, Xu SG, et al. Pure red cell aplasia due to parvovirus B19 infection after liver transplantation: a case report and review of the literature. World J Gastroenterol. 2007;13(13):2007–10. doi: 10.3748/wjg.v13.i13.2007
- Kurtzman G, Frickhofen N, Kimball J, Jenkins DW, Nienhuis AW, Young NS. Pure red-cell aplasia of 10 years’ duration due to persistent parvovirus B19 infection and its cure with immunoglobulin therapy. N Engl J Med. 1989;321(8):519–23. doi: 10.1056/NEJM198908243210807
