Abstract
Objectives: Imatinib is a cornerstone of treatment of chronic myeloid leukemia. It remains unclear whether transient treatment discontinuation or dose changes affect outcome and this approach has not yet been approved for use outside clinical trials.
Methods: We conducted a retrospective single-institution observational study to evaluate factors affecting response in ‘real-life’ clinical practice in 138 chronic myeloid leukemia patients in chronic phase treated with imatinib. We used a novel longitudinal data analytical model, with a generalized estimating equation model, to study BCR–ABL variation according to continuous standard dose, change in dose or discontinuation; BCR–ABL transcript levels were recorded. Treatment history was subdivided into time periods for which treatment was given at constant dosage (total 483 time periods). Molecular and cytogenetic complete response was observed after 154 (32%) and 358 (74%) time periods, respectively.
Results: After adjusting for length of time period, no association between dose and cytogenetic complete response rate was observed. There was a significantly lower molecular complete response rate after time periods at a high imatinib dosage.
Discussion: This statistical approach can identify individual patient variation in longitudinal data collected over time and suggests that changes in dose or discontinuation of therapy could be considered in patients with appropriate biological characteristics.
Introduction
For many years, imatinib mesylate (IM), an oral tyrosine kinase inhibitor (TKI) of the rearranged BCR–ABL, has represented the standard-of-care in the front-line treatment of chronic phase-chronic myeloid leukemia.Citation1–Citation3 The majority of patients with newly diagnosed CML treated with IM achieve complete cytogenetic responses (CCyR), namely absence of Philadelphia-positive cells in at least 20 bone marrow metaphase cells, within 12 months or more of starting therapy; this is the only factor significantly affecting overall survival (OS).Citation2 Moreover, life expectancy is believed to be similar to that of individuals with other ‘chronic diseases’, such as hypertension or diabetes. Now two even more potent oral BCR–ABL inhibitors, dasatinib (DAS) and nilotinib (NIL), initially approved by the US FDA for patients with refractory disease or intolerance to IM, have been approved as first-line therapy due to excellent results in terms of cytogenetic and molecular responses in the DASISION and ENESTnd studies.Citation4,Citation5 Both NIL and DAS can obtain a quicker major molecular response (MMR) or complete molecular response (CMR: no detectable BCR–ABL mRNA). However, we do not know what effect, if any, this quicker response has on OS or on the risk of poor cytogenetic and molecular response.Citation6
Moreover, it is still not clear which level of molecular response (MR: MR3, MR4, MR4.5, or CMR) should be the target of therapy because OS and progression free survival, in our clinical experience, seem equally good, despite the fact that it has been reported that also deep molecular response significantly affects survival.Citation7 Also, as the sensitivity of real-time quantitative reverse transcriptase-based methods (RT-QPCR) for the monitoring of CML has improved over time,Citation8–Citation10 it has been seen that achievement of clinical, morphological, and cytogenetic remission does not indicate eradication of the disease.Citation11–Citation14 A CMR represents a deeper level of response, but it does not guarantee disease eradication because it depends on the detection limit of the assay used. For months or years after achieving a CCyR, the majority of CML patients have measurable disease by RT-QPCR and would relapse if treatment were withdrawn.Citation12 In fact, there is still no consensus about the need to eradicate disease.Citation7
In clinical practice, CML patients outside of trials are assessed for possible TKI dose modification because of age, toxicity, compliance, or increase in BCR–ABL level.Citation15–Citation18 In a multivariate analysis, including most known biological prognostic factors, patient adherence was the only independent predictor for achievement of CCyR and CMR.Citation19 So dose changes or discontinuation of therapy are attractive but need to be carefully managed and have not yet been approved for use outside clinical trials.
We conducted a single-institution retrospective observational study on CML patients treated with imatinib to evaluate factors affecting response in ‘real-life’ clinical practice using a statistical method rather than biological findings, as suggested by the mathematical model of Olshen et al.Citation20 In fact, conventional statistical methods use a collective presentation of data, e.g. QPCR level, percentage, or type of mutations. We aimed to construct a dynamic model in which each patient was his or her own control. Continuous dose or any changes in dose and/or discontinuation of therapy together with BCR–ABL transcript levels were recorded during patients’ follow up. We, therefore, applied a longitudinal data analytical model to determine whether intermittent IM therapy can be used in patients with CML. In particular, we assessed whether the probability of a CCyR or an MR, and probability of disease progression after a period of IM treatment at constant standard dosage were influenced by dosage and/or duration of treatment.
Patients and methods
Patients
A total of 138 patients, treated with IM from 2000 to 2013 with regular follow up were available for statistical analysis as of September 2013. All data captured were accurately taken visit by visit and recorded in the patients’ clinical files. Patients’ characteristics are as shown in Table .
Table 1 Patients’ characteristics
These patients had been diagnosed between 1989 and 2013, with the exception of two patients diagnosed in 1978 and 1985; none of these patients were in a trial at the time of analysis. Patients had given informed consent within our treatment procedures. All procedures followed were in accordance with the institutional and national ethical standards on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. First-line therapy was 72 patients with IM, 27 with interferon (IFN), 37 with hydroxyurea (HU), 2 with NIL. Forty-nine patients received IM as second-line therapy. Among these, 23 had previously been treated with IFN. The relative quantification of BCR–ABL transcript was initially performed as previously described,Citation10,Citation21 and thereafter according to the current International Scale.
IM dosage, response, disease progression, and any treatment discontinuation were retrieved from medical records and longitudinally recorded. Each patient's follow up was subdivided into time periods of treatment at constant dose and/or time periods of therapy discontinuation. Cytogenetic progression was defined as the loss of CCyR. The molecular progression was considered when the patient lost MMR confirmed by two consecutive QPCR samples.
We analyzed a total of 483 time periods of IM therapy with a constant dose, coded into three groups: low dose (100–300 mg/day), standard dose (400 mg/day), high dose (>400–800 mg/day), and response was evaluated at the end of each treatment period. We also studied the outcome of different time periods during which therapy was suspended: >1 month (mos), >12 months.
Statistical analysis
Categorical variables were described by count and relative frequency according to dosage group and length of time period, and numerical variables by median and range. Association between categorical variables was tested by the two-tailed Fisher's exact test for two-way tables. Differences in numerical variables between two groups were tested by the non-parametric Wilcoxon's rank-sum test. We applied generalized estimating equation (GEE) modelsCitation22 for the analysis of longitudinal data to study the BCR–ABL variation of each patient according to dose change to pick up individual patient variation. We considered the following variables as longitudinal outcomes: molecular and cytogenetic response, molecular and cytogenetic progression. Since the data consist of clinical, molecular, and cytogenetic information repeatedly collected over time for each subject, these models were adopted to properly account for the panel structure of the data. In particular, GEE logistic regression models were used to assess the association between outcome (molecular/cytogenetic response and progression) and treatment dose and duration, and were designed to account for correlation due to repeated measurements during follow up of each single patient.
Model fit was assessed with the quasi-likelihood under the independence (QIC) model criterion.Citation23,Citation24 Statistical analyses were carried out using Stata 12.1 (StataCorp LP, USA).
Results
Changes in dosage and treatment
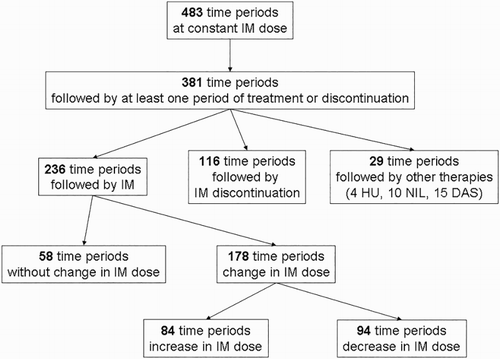
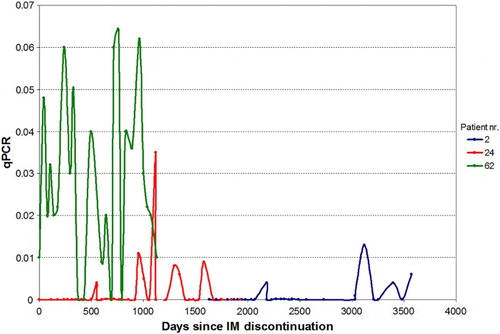
IM was administered in 483 time periods. Changes in dosage and treatment are as shown in Fig. . Fifty-five patients (38.7%) discontinued IM at least once. Among these, 37 patients (67.3%) stopped IM therapy for >1 month due to toxicity, low compliance or by shared patient/physician decision. IM therapy was re-started in 32 of these patients. Twelve of 55 patients (21.8%) suspended therapy for >12 months having achieved CMR or MMR. Of these, five have not re-started therapy because of sustained CMR or MMR (Fig. ; for clarity, Patients 51 and 74 have not been included in the graph because all their readings are zero).
Figure 2 Sequential evaluations of BCR–ABL level in three of the five patients who discontinued IM for at least 12 months and who are still off treatment. For clarity, patients 51 and 74 have not been included in the graph because all their readings are zero
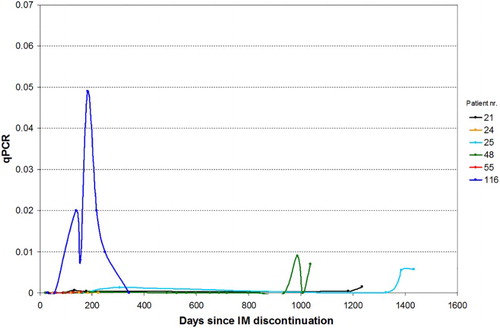
Seven patients re-started therapy due to a cautionary approach on the part of the curing physician in consideration of the fluctuating QRT-PCR values; interestingly, they showed the same biological behavior as the five patients who have not re-started therapy (Figs. and ).
Figure 3 Sequential evaluations of BCR–ABL level in six patients who discontinued IM for at least 12 months and who then re-started treatment. Patients with only one qPCR measurement have not been included in the graph
At the time of statistical analysis of the 138 study patients, 102 (74%) were on therapy with IM, 14 (10%) with NIL, and 15 (11%) with DAS; 7 patients (5%) were off therapy.
Changes in dosage and treatment according to previous IM dosage
Therapy characteristics in the period following the treatment with IM according to dosage are as shown in Table .
Table 2 Therapy characteristics in the period following the treatment with imatinib according to dosage
Considering therapy changes after a time period at low dose, 37% discontinued treatment, and 2.4% changed TKI. In the standard-dose group, 35.7% discontinued IM therapy and 9.5% changed TKI. In the high-dose group, 6.8% discontinued IM therapy and 11.9% changed TKI (Table ).
After a period at standard dose, 54.1% of patients’ time periods showed a reduced dose due to low compliance or toxicity, and 42.9% showed an increased dosage because of suboptimal response (according to Guidelines at the time of evaluation) (Table ). After a period at low dosage, there was no change in dose group in 45.5% of time periods, due to patient frailty, low compliance or toxicity, while maintaining a good response; 50.7% subsequently changed to standard dose and 3.9% to high dose. After a time period at high dose, dosage was reduced to standard in 67.2% of patients’ time periods (Table ).
Response to treatment
Considering cytogenetic response after a period of IM treatment at constant dose, 74.3% of time periods resulted in a CCyR and 6.2% showed no CyR; 19.5% showed MCyR + PR. In terms of MR, 31.9% of time periods resulted in CMR, 29.6% in MMR (MR3, MR4, MR4.5), and 35.6% in a suboptimal response, while 2.9% showed no response. Considering CMR and MMR together, 61.5% of the 483 time periods showed a response.
Duration of treatment periods was significantly longer for time periods leading to a CCyR (median 455 days) than for periods that ended before CCyR was achieved (median 57 days; P < 0.001). In terms of MR, the time periods that ended with a CMR or an MMR were longer (median 545 days) than periods showing a suboptimal or no response (median 78 days; P < 0.001).
Response to treatment in the period following IM according to dosage is as shown in Table .
Table 3 Response to treatment in the period following imatinib according to dosage
Periods at standard dose showed a significant higher response rate, both when considering cytogenetic response and when considering molecular response (Table ). Importantly, after adjustment for duration of treatment period and accounting for the panel structure of the data (i.e. repeated assessments during follow up of each patient) by applying a multivariate GEE logistic model, dose lost significance, and duration of treatment was the only significant predictor of CCyR (P < 0.001). By applying the same model to the analysis of molecular response, the reduction in CMR rate after periods at high dose compared with periods at standard dose remained significant (P = 0.025) also after accounting for treatment duration. Statistical significance of the change in response rate according to dosage is as shown in Table .
In addition, in a multivariate GEE logistic model with dose category, duration of treatment period, and previous suspension as covariates, both duration and previous suspension maintained a positive significant association to CCyR rate (Table ).
Disease progression
Regarding cytogenetic progression, a comparison between those patients receiving IM as first-line therapy with those who received IM as second-line therapy showed that 9.7% vs. 22.5% (P = 0.069, borderline significance) showed cytogenetic progression. Comparison between those patients receiving IM as first-line therapy compared with those received IM as second-line therapy showed that 31.9% vs. 34.7% (P = 0.0844, NS) showed molecular progression.
The overall cytogenetic progression rate at the end of a period of IM treatment was 7.1% while the molecular progression rate was 12.8%. Treatment dose was not significantly associated either to cytogenetic or to molecular progression rate (Table ).
Table 4 Cytogenetic and molecular progression according to imatinib dosage
The median duration of treatment in patients who did not experience molecular progression was 259 days, while the median duration of treatment in patients who had a progression was 281 days (P = 0.822). Considering cytogenetic progression, the time periods resulting in a progression had a median duration of 360 days vs. 250 days for periods with no progression detected (P = 0.447).
This result was confirmed by multivariate GEE logistic regression analysis in which neither dose nor duration of treatment were significant predictors of cytogenetic or molecular progression. When adding type of treatment in the previous period (IM, other drug, no treatment) as a covariate in the regression models, there was a significantly lower molecular progression rate in the treatment period preceded by at least 1 month of discontinuation (P = 0.011).
Discussion
Published data support the safety of IM dose reduction or an ‘on/off’ therapy,Citation15–Citation18,Citation21,Citation25–Citation29 but so far these studies have not led to guidelines being drawn up for this purpose. The current estimated IM stopping rate after stable CMR is approximately 40%, although this percentage may change over time as the eligibility criteria for TKI discontinuation and resumption are refined.Citation30
The intrinsic capacity of any residual leukemic cells to proliferate following the withdrawal of treatment may be important, but immunological suppression of the leukemic clone may also play a role.Citation31 Treatment can lead to low-level persistence of CML stem cells, assuming that these cells are less susceptible to drug-mediated activity;Citation13,Citation14 this might explain why the disease tends to relapse after treatment discontinuation even with no acquired drug resistance.Citation32
The French Stop Imatinib (STIM) and TWISTER trialsCitation25,Citation27 reported that IM was safely discontinued in 40% with at least 2 years of CMR. Studied factors included gender, Sokal score, length of IM treatment. In the STIM trial, only total duration of IM therapy and Sokal score were significant predictors of relapse risk. Length of IM treatment period seemed to annul the significance of the Sokal score as a predictor of OS or PFS, with the exception of high-risk patients.Citation33
Our study is characterized by a novel statistical approach: data consist of clinical molecular and cytogenetic information repeatedly collected over time for each subject, applying GEE modelsCitation22 to analyze longitudinal data to pick up individual patient variation. In particular, GEE logistic regression models were used to assess the association between outcome (molecular/cytogenetic response and progression) and treatment dose and duration. After adjusting for time period duration, dose lost significance, and treatment duration was the only significant predictor of cytogenetic complete response (P < 0.001).
The non-randomized approach, together with the long study period (2000–2013) and technological changes in RT-QPCR assessment, may not allow an efficient comparison of patients’ response in different time periods to be made. However, there is no clear difference in patient outcome (CMR and OS) when using either the older or the more recent technique to assess MR.Citation8–Citation10,Citation34 We must also consider that pre-treatment with IFN in good survivors might have influenced remission stability when compared with first-line imatinib-treated patients. Although our results are in keeping with the recent update on results of BCR–ABL monitoring,Citation33,Citation35 further studies in ‘real-life’ situations are needed before reliable medical conclusions can be drawn.
Molecular response to IM in CML is associated with a biphasic but heterogeneous decline in BCR–ABL transcript levelsCitation36 that could explain the different outcome in each patient and a divergent response to events such as dose changes, interruption, changes in TKI therapy. Results from the STIM2 trialCitation29 demonstrated that 33% of patients off therapy showed a fluctuation in BCR–ABL1 transcript levels from CMR (0%) to MMR (<0.1%), despite the fact that patients did not re-start IM. Interestingly, we observed the same phenomenon in our series of patients.
In our study, the probability of cytogenetic and of molecular progression after a period of treatment with IM were not significantly associated to dose and length of period. In addition, there is no statistically significant difference in molecular progression between those patients who received IM as first-line therapy compared with second-line therapy. The probability of molecular progression was significantly lower (P = 0.011) when the previous period was one of IM suspension, suggesting that the biology of the disease is not very aggressive and the patient could suspend IM without affecting outcome. Interestingly, the percentage of molecular response after a period of treatment with IM at constant standard dosage is similar to the percentage of patients obtaining molecular response reported in literature,Citation2,Citation7 confirming that the proposed statistical model can adequately pick up the biological behavior of patients. A longer treatment period was associated to a higher response rate, reflecting and explaining the contribution of those patients who are late responders.
A non-CMR to standard IM dose often leads to the clinical decision to increase dosage, but it should be remembered that a non-CMR might be due to a resistance to the drug rather than to the fact that the patient is a late responder. This suggests that if CMR is not reached after a period at standard dose, changing TKI should be considered as an alternative to increasing IM dosage. The possibility of varying dosage is also considered in ‘real-life’ patient clinical management.Citation15–Citation17 In our study, 62% of time periods were followed by a dose modification, but there was no substantial difference in outcome from the published data.Citation2,Citation7 In univariate analysis, periods at standard dose showed a significantly higher response rate both when considering CCyR (after low, standard, or high dose) and when considering molecular relapse (after low, standard, or high dose). In multivariate analysis, duration of treatment period at constant dose was the only factor that retained statistical significance. The change in response rate according to dosage was not significant when considering CMR or MMR as outcome and adjusting for treatment duration.
In our series, characterized by a ‘real-life’ approach, out of the 12 patients who suspended therapy because of sustained (>12 months) CMR or MMR, seven re-started IM therapy due to 1-log increase of BCR–ABL transcript confirmed at two consecutive checkups, though these patients maintained MMR or CMR. It is intriguing that the BCR–ABL level of the five patients who have not re-started therapy have not always had undetectable PCR during the observation period (Fig. ). Figure shows sequential evaluations of BCR–ABL level in six patients who discontinued IM for at least 6 months and who then re-started treatment.
Conclusions
These data, picked up in a ‘real-life’ situation, suggest the intriguing concept that it may not be necessary to reach a CMR in order to change dose or discontinue IM therapy. We do, however, agree with Yilmaz and JabbourCitation37 that discontinuation is discouraged outside of a clinical trial. The prompt response to resumed IM therapy, together with a likely improvement in quality of life while off therapy (S. Merante, personal observations, 2016), suggest that patients (especially the elderlyCitation18) who have sustained CMR or MMR may be candidates for an ‘on-off’ approach.Citation12,Citation13 It must be emphasized that a ‘real-life’ approach requires strict follow up and studies are still ongoing. The results of our statistical approach are useful to pick up individual patient variation in longitudinal data collected over time. We suggest that this approach could be adopted in future ‘real-life’ observational studies to further investigate continuous standard dosage, dose changes, and the length of time for which these were maintained, and discontinuation in IM or second-generation TKI.
Acknowledgements
Our thanks to Anne Freckleton for her help in preparing this manuscript.
Disclaimer statements
Contributors All authors have made substantial contributions to the conception and design of the study, acquisition of data, or analysis and interpretation of the data, drafting the article or revising it critically for important intellectual content, and have given their final approval for the version to be submitted.
Funding None.
Conflicts of interest None.
Ethics approval None.
References
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401
- Kantarjian H, O'Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single institution historical experience. Blood 2012;119:1981–7. doi: 10.1182/blood-2011-08-358135
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457
- Hochhaus A, Shah NP, Cortes J, Baccarani M, Bradley-Garelik MB, Dejardin D, et al. Dasatinib versus imatinib (IM) in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): DASISION 3-year follow-up. J Clin Oncol. 2012;30:6504.
- Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim D-W, et al. Nilotinib versus imatinib in patients with newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP): ENESTnd 36-month follow-up. Leukemia 2012;26:2197–203. doi: 10.1038/leu.2012.134
- Marin D. Initial choice of therapy among plenty for newly diagnosed chronic myeloid leukemia. Hematol Am Soc Hematol Educ Progr. 2012:115–21.
- Marin D. Patient with chronic myeloid leukemia in complete cytogenetic response: what does it mean, and what does one do next?. J Clin Oncol. 2014;32(5):379–84. doi: 10.1200/JCO.2013.52.9230
- Cross NC, White HE, Muller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012;26:2172–5. doi: 10.1038/leu.2012.104
- Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006;108:28–37. doi: 10.1182/blood-2006-01-0092
- Gabert J, Billard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of “real time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual d ase detection in leukemia – a Europe against cancer program. Leukemia 2003;17:2318–57. doi: 10.1038/sj.leu.2403135
- Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia 2010;24:1719–24. doi: 10.1038/leu.2010.185
- Cullis JO, Marks DI, Schwarer AP, Barrett AJ, Hows JM, Swirsky DM, et al. Relapse into blast crisis following bone marrow transplantation for chronic phase chronic myeloid leukaemia: a report of five cases. Br J Haematol. 1992;81:378–82. doi: 10.1111/j.1365-2141.1992.tb08243.x
- Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–30. doi: 10.1200/JCO.2012.48.5797
- Horn M, Glauche I, Müller MC, Hehlmann R, Hochhaus A, Loeffler M, et al. Model-based decision rules reduce the risk of molecular relapse after cessation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Blood 2013;121(2):378–84. doi: 10.1182/blood-2012-07-441956
- Efficace F, Baccarani M, Breccia M, Alimena G, Rosti G, Cottone F, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood 2011;118(17):4554–60. doi: 10.1182/blood-2011-04-347575
- Latagliata R, Breccia M, Carmosino I, Cannella L, De Cuia R, Diverio D, et al. “Real-life” results of front-line treatment with imatinib in older patients (≥65 years) with newly diagnosed chronic myelogenous leukemia. Leuk Res. 2010;34:1472–5. doi: 10.1016/j.leukres.2010.07.001
- Gugliotta G, Castagnetti F, Apolinari M, Pirondi S, Cavo M, Baccarani M, et al. First-line treatment of newly diagnosed elderly patients with chronic myeloid leukemia: current and emerging strategies. Drugs 2014;74:627–43. doi: 10.1007/s40265-014-0207-7
- Russo D, Martinelli G, Malagola M, Skert C, Soverini S, Iacobucci I, et al. Effects and outcome of a policy of intermittent imatinib treatment in elderly patients with chronic myeloid leukemia. Blood 2013;121(26):5138–44. doi: 10.1182/blood-2013-01-480194
- Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in chronic myeloid leukemia patients who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;24:2381–88. doi: 10.1200/JCO.2009.26.3087
- Olshen A, Tang M, Cortes J, Gonen M, Hughes T, Branford S, et al. Dynamics of chronic myeloid leukemia response to dasatinib, nilotinib, and high-dose imatinib. Haematologica. Doi:10.3324/haematol.2014.085977
- Merante S, Orlandi E, Bernasconi P, Calatroni S, Boni M, Lazzarino M. Outcome of four patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica 2005;90:979–81.
- Liang K-Y, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13
- Pan W. Akaike's informat ion criterion in generalized estimating equations. Biometrics 2001;57:120–5. doi: 10.1111/j.0006-341X.2001.00120.x
- Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7(2):209–20.
- Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Intergroupe Francais des Leucemies Myeloides Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least two years: the prospective, multicentre, Stop IMatinib (STIM) trial. Lancet Oncol. 2010;11:1029–35. doi: 10.1016/S1470-2045(10)70233-3
- Cortes J, O'Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood 2004;104:2204–5. doi: 10.1182/blood-2004-04-1335
- Ross DM, Branford S, Seymour JF, Arthur C, Yeung DT, Dang P, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013;122:515–22. doi: 10.1182/blood-2013-02-483750
- Koskenvesa P, Kreutzman A, Rohon P, Pihlman M, Vakkila E, Räsänen A, et al. Imatinib and pegylated IFN-a2b discontinuation in first-line chronic myeloid leukemia patients following a major molecular response. Eur J Haematol. 2014;92(5):413–20. doi: 10.1111/ejh.12258
- Mahon FX, Nicolini FE, Noël MP, Escoffre M, Charbonnier A, Rea D, et al. Preliminary report of the STIM2 study: a multicenter stop imatinib trial for chronic phase chronic myeloid leukemia de novo patients on imatinib. Blood 2013. ASH Annual Meeting Abstract 654.
- Ross MD, Hughes TP. How I determine if and when to recommend stopping tyrosine kinase inhibitor treatment for chronic myeloid leukaemia. Brit J Haematol. 2014;1:3–11. doi: 10.1111/bjh.12892
- Wodarz D. Heterogeneity in chronic myeloid leukaemia dynamics during imatinib treatment: role of immune responses. Proc Royal Soc Biol. 2010;277:1875–80. doi: 10.1098/rspb.2009.2179
- Wodarz D. Stem cell regulation and the development of blast crisis in chronic myeloid leukemia: Implications for the outcome of Imatinib treatment and discontinuation. Med. Hypotheses 2008;70:128–36. doi: 10.1016/j.mehy.2007.03.040
- Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32. doi: 10.1056/NEJMoa030513
- Malagola M, Breccia M, Skert C, Cancelli V, Soverini S, Iacobucci I, et al. Long term outcome of Ph+CML patients achieving complete cytogenetic remission with interferon based therapy moving from interferon to imatinib era. Am J Hematol. 2014;89(2):119–24. doi: 10.1002/ajh.23593
- de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–63. doi: 10.1200/JCO.2007.15.8154
- Branford S, Seymour JF, Grigg A, Arthur C, Rudzki Z, Lynch K, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007;13:7080–5. doi: 10.1158/1078-0432.CCR-07-0844
- Yilmaz M, Jabbour E. Tyrosine kinase inhibitors early in the disease course: lessons from chronic myelogenous leukemia. Semin Oncol. 2015;42(6):876–86. doi: 10.1053/j.seminoncol.2015.09.030