Abstract
Objectives: LEUKOSPECT was a retrospective, multicenter, epidemiologic study carried out in Russia and Ukraine, aiming to assess the prevalence and cumulative 5-year incidence of chronic lymphocytic leukemia (CLL) in the adult population.
Methods: All data were collected manually from patient medical records at each of the study sites and from official censuses. CLL prevalence and incidence were determined from a count of CLL cases (previously diagnosed and new cases) in the population who made at least one clinic visit for CLL during the period from 1 January 2009 to 31 December 2013.
Results: The recorded cumulative 5-year incidence of CLL during the study ranged from 1.46 per 100 000 persons (95% CI: 0.85–2.34) in Yekaterinburg, Russia, to 4.34 per 100 000 persons (95% CI: 2.48–7.04) in Luhansk, Ukraine. In 2013, the lowest prevalence of CLL was also recorded in Yekaterinburg: 7.11 per 100 000 persons (95% CI: 5.67–8.81). This was approximately 3.1 times lower than in Luhansk (21.92 per 100 000 population; 95% CI: 17.38–27.28).
Conclusion: The results of this study show diverse CLL incidence and prevalence patterns in the adult population of the Russian Federation and Ukraine. Authors propose a more comprehensive study with large region involvement to provide a more precise description of the incidence and prevalence of CLL in Eastern European countries and to better understand disparities reported versus the USA and other Western countries.
Introduction
Chronic lymphocytic leukemia (CLL) often occurs during or after middle age and is the most common leukemia in Western countries, accounting for approximately 30% of all leukemias in the USA.Citation1 The disorder is more common in men with a male-to-female ratio of approximately 1.9:1.Citation2
The majority of epidemiology studies of CLL have been conducted in Western Europe and the USA. The incidence rates among men and women in the USA are approximately 6.2 and 3.2 cases per 100 000 persons per year, respectively.Citation2 In Europe, these incidence rates are 5.87 and 4.01 cases per 100 000 persons per year, respectively.Citation3 Results of the study conducted by Watson et al.Citation4 showed that in 27 European states plus Iceland, Norway, and Lichtenstein, 1- and 5-year CLL partial prevalence estimates totaled approximately 13 952 and 46 633 individuals, respectively, in 2006. In Asia, incidence and prevalence rates of hematologic malignancies are lower than those in Europe and the USA.Citation5
In many countries the epidemiology of CLL remains unknown; in Russia and other Eastern European countries, no information on the prevalence or incidence of CLL has been published to date, and no registries covering populations of entire countries have been created. According to official statistics, in Russia, the standardized incidence rate of CLLs (diagnosis code C91.1–9) in 2012 was 1.6 (2.3 in men and 1.2 in women) per 100 000. The epidemiologic data vary widely between regions;Citation6 for example, in Ukraine in 2008, the CLL incidence was 3.3 per 100 000.Citation7 Another problem is that there are no special programs for leukemia screening; only following a blood analysis performed at a patient's routine visit may the CLL diagnosis be suspected.
This study, LEUKOSPECT (GlaxoSmithKline protocol # 117047), was the first epidemiologic study of CLL aimed at assessing the prevalence (in 2013) and cumulative 5-year (2009–2013) incidence of CLL in the adult population of cities in two countries – the Russian Federation (Russia) and Ukraine.
Material and methods
Study design
LEUKOSPECT was a retrospective, multinational, multicenter, epidemiologic study carried out across two post-Soviet countries – Russia and Ukraine. Three specialized hematologic and/or oncologic centers have captured epidemiologic data by qualified hematologists and oncologists. Two centers in Russia (Yekaterinburg and Khabarovsk) and one site in Ukraine (Luhansk) were included in the final analysis of the epidemiology of CLL. These three cities were selected based on the feasibility of reliably capturing all CLL cases. As the expected incidence of the disease is low, data were collected for 5 years rather than 1 year (2009–2013).
The study was conducted in accordance with local legislation and ethical requirements. Informed consent was obtained from all patients in compliance to consent process reviewed by relevant ethics committees.
Study area and population
Study cities were selected based on the following: (1) they have a relatively well demarked population; (2) patient referral patterns are well characterized; and (3) the population makeup is reasonably representative of the country as a whole (for example, did not over-represent an ethnic minority group).
The size of the population was obtained from local official census data in each city. If data for age/sex groups were absent, they were extrapolated from age/sex distribution from the whole population in the appropriate region (i.e. Luhansk Oblast, Sverdlovsk Oblast, or Khabarovsk Krai). Sex/age distribution for population was calculated using the proportion of males/females in the adult population.
Official adult population data were available on 01 January of each year. Therefore, it was assumed that the population on 31 December 2009 was equal to the same on 01 January 2010; population on 31 December 2010 was equal to 01 January 2011, etc. All adult population data, summarized per study years and cities, are as available in Table .
Table 1 Summary of the total adult population characteristics
Ascertainment of CLL cases
Trained medical staff hand-searched clinical records to identify all adult patients with CLL diagnoses established based on recommended criteria: ≥5 × 109 B-lymphocytes/l in the peripheral blood and clonality of the circulating B-lymphocytes confirmed by flow cytometry.Citation8 All patients’ data were abstracted from the medical records.
Patients’ count rules were the following:
Incident CLL patient – CLL diagnosis established from 01 January 2009 to 31 December 2013.
Prevalent CLL patient – CLL diagnosis established before 31 December 2013.
Prevalent cases – all adult patients identified from medical records with a CLL diagnosis dated before 31 December 2013, reside in the target city, and are not deceased on this date.
Incident cases – all adult patients identified from medical records who were newly diagnosed with CLL between 01 January 2009 and 31 December 2013, and resided in the target city at the date of diagnosis.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics software (IBM Corp., New York, NY, USA) version 21.0. Descriptive analyses (proportions, mean, standard deviation, confidence intervals [CI] for means) were performed for each country.
Calculations were performed as follows:
Prevalence estimate – annual mean value of all individuals diagnosed with CLL from 01 January 2009 to 31 December 2013, who were resident in the target city during this period/mean of total adult population for the period of 2009–2013.
Incidence estimate in 2013 – annual mean value of all individuals newly diagnosed with CLL between 01 January and 31 December 2013, who were resident in the target city at the time of diagnosis/mean of total adult population for the period of 2009–2013.
Results and discussion
Study population and baseline characteristics
According to census data, the biggest adult population in participating cities was in Yekaterinburg – the total city adult population ranged from 1 124 480 (in 2009) to 1 180 721 (in 2013). The lowest adult population was in Luhansk – ranging from 374 089 (in 2009) to 365 003 (in 2013) (Table ).
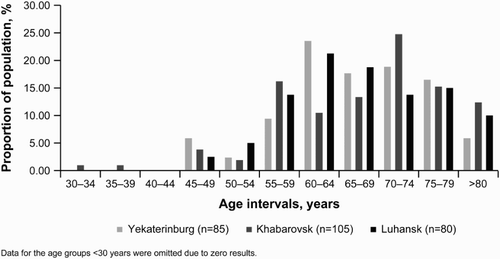
Baseline characteristics of incident cases in the adult population are as presented in Table and Fig. , and for prevalent cases – in Table and Fig. . It should be noted that all study participants were of Caucasian race, and therefore ethnicity-adjusted calculations were not performed.
Figure 2 Age intervals and population proportions of prevalence cases at the end of data collection.
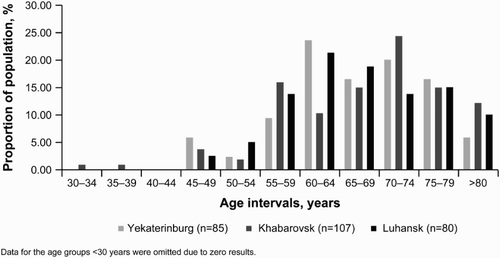
Table 2 Baseline characteristics of CLL incidence cases in the adult population (cumulative 5-year incidence)
Table 3 Baseline characteristics of CLL prevalence cases in the adult population
Cumulative 5-year (2009–2013) incidence of CLL in the adult population
In Yekaterinburg, the annual mean number of incidence cases between 2009 and 2013 was 16.80. Local cumulative incidence of CLL in this 5-year period among the adult population was 1.46 per 100 000 persons (Table ).
Table 4 Cumulative 5-year (2009–2013) incidence of CLL among adult populations
In Khabarovsk and in Luhansk, the cumulative incidence rates of CLL for 2009–2013 among the adult population were 4.32 and 4.34 per 100 000 persons, respectively. Thus, it could be stated that a cumulative incidence of CLL in Yekaterinburg, among the adult population in a 5-year period, is almost three times lower than in other cities that participated in this study.
The recorded incidence rates for the 5-year period obtained in this study are similar or even lower than the values described in the literature for Russia; in the Republic of Bashkortostan, the incidence rate of all hemoblastosis types for the 5-year period 2004–2008 was 9.55 per 100 000 persons.Citation9 Published data available for some European countries show higher values than were obtained in our study; in the Czech Republic in the years 2006 and 2007, the incidence of CLL was 5.8 and 6.2, respectively.Citation10
Cumulative 5-year (2009–2013) sex-adjusted incidence of CLL in the adult population
The lowest values for the incidence of CLL among the adult population in 2009–2013 were recorded in Yekaterinburg: 1.79 and 1.20 per 100 000 persons in the male and female population, respectively. The incidence of CLL was also more common in males, with a male-to-female ratio of approximately 1.49 (Table ).
Table 5 Cumulative 5-year (2009–2013) sex-adjusted incidence of CLL in adult populations
The maximum values of the local cumulative incidence of CLL in 2009–2013 were recorded in Khabarovsk: 5.06 in the male population and 3.67 per 100 000 persons in the female population.
Cumulative 5-year (2009–2013) age-adjusted incidence of CLL in the adult population
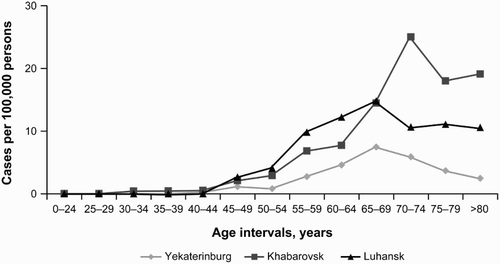
The highest mean cumulative CLL incidence was recorded in Khabarovsk in the age groups 70–74 years old – 25.00 (95% CI, 8.57–56.61), and >80 years old – 19.08 per 100 000 persons (95% CI, 2.67–65.36), in the adult population for both sexes (Fig. ). In the age groups <65 and ≥65 years old, the obtained values for the cumulative incidence were 1.57 (95% CI, 0.69–3.06) and 19.46 (95% CI, 10.25–33.56), respectively.
The lowest mean cumulative CLL incidence was recorded in Yekaterinburg. For comparison, in the age group 70–74 years old, the cumulative CLL incidence was only 5.88 per 100 000 persons (95% CI, 1.03–18.45) for both sexes, which is more than four times lower than the value recorded in the same age group in Khabarovsk. In the age groups <65 and ≥65 years old, the obtained values for the cumulative incidence were 0.70 (95% CI, 0.31–1.35) and 4.89 (95% CI, 2.16–9.48) per 100 000 persons, respectively.
For comparison, the available published data on lymphoid malignancies (LMs) registered in 2000–2002 by 44 European cancer registries showed age-standardized incidence rates for small B-cell lymphocytic lymphoma (SBLL) and CLL of 3.79 per 100 000 persons and 24.5 per 100 000 for all LMs.Citation3 For a similar time period in the USA, 17 Surveillance, Epidemiology, and End Results program (SEER) registries showed higher age-standardized incidence rates for SBLL/CLL (5.10 per 100 000 persons), as well as for all LMs (33.42 per 100 000 persons) for the years 2001–2003.Citation6,Citation11,Citation12
The peak incidence values in the 70–74 years age interval, recorded in Khabarovsk (the most eastern city in the study, and near China and North Korea), have reached the cumulative rate of 25.00 cases per 100 000 persons (95% CI, 8.57–56.61); this is much higher than in Western study sites. Even considering the fact that in Asia the incidence and prevalence rates of hematologic malignancies are lower than those in Europe and the USA (in South Korea from 1999–2008, the age-standardized incidence rates increased from 10.2 to 13.7 per 100 000 persons),Citation13 our study was conducted only in Caucasian patients. Therefore, this eliminates any possible explanations of such disparities that could be possibly caused by the environment or genetic factorsCitation14,Citation15 for different ethnic groups (especially Asian) that have been widely discussed in the literature in the last decade.
In addition, the results of this study revealed a trend to the lower incidence rate, after the age of 74 years, for the cumulative age-adjusted CLL incidence for the 5-year period in comparison with the 65–69 years age interval in all study sites that participated in the study (Fig. ). However, it has been reported that the incidence of CLL increases with age in all race and sex subgroups, reaching a maximum at between 80 and 84 years of age, according to 18 SEER registries (2008–2012).Citation2 The only Russian cancer prevalence register available also displays stable high incidence rates in the population aged over 70 years for the diagnosis ‘other leukemias (including CLL)’ (ICD-10 code С91.1-9) at the level of 0.79–0.82 per 100 000 persons.Citation16
Cumulative 5-year (2009–2013) prevalence of CLL in the adult population and prevalence in 2013
The lowest cumulative 5-year (2009–2013) CLL prevalence was in Yekaterinburg – just 3.98 per 100 000 persons, almost 3.4 times lower than in Khabarovsk, and 3.0 times lower than in Luhansk – at 13.46 and 11.88 per 100 000 persons, respectively (Table ).
Table 6 Cumulative 5-year (2009–2013) prevalence of CLL among adult populations
In 2013, the lowest prevalence of CLL was also recorded in Yekaterinburg – 7.11 per 100 000 persons; this was about 3.1 times lower than in both Khabarovsk and Luhansk (21.88 and 21.92 per 100 000 persons, respectively).
One of the reasons for this discrepancy could be differences in regional oncology healthcare practices, which is supported by the fact that an epidemiology study of CLL in Manitoba, Canada, with additional immunophenotyping and subsequent comparison with the cancer registry for the period 1998–2003, revealed that out of 616 cases identified, 27% of patients identified by flow cytometry were not on the cancer registry.Citation17 In contrast, in the Republic of Bashkortostan, age-standardized prevalence of CLL in the years 1999–2008 was 21.97 per 100 000 persons for both sexes in patients aged >60 years.Citation9 However, despite those facts, a city selection bias for the study site location in our study cannot be ruled out.
Cumulative 5-year (2009–2013) sex-adjusted prevalence of CLL in the adult population
An overall tendency for the sex-adjusted CLL prevalence was the same as for the cumulative CLL prevalence: the lowest values were in Yekaterinburg for both males and females – 4.62 and 3.48 per 100 000 persons, respectively (Table ).
Table 7 Sex-adjusted 5-year (2009–2013) prevalence of CLL in adult populations
Prevalence values of CLL in the female population of Khabarovsk and Luhansk were almost equal (11.49 and 11.55 per 100 000, respectively). The highest CLL prevalence value was recorded in the Khabarovsk male population – 15.70, which was 3.4 times higher than in the Yekaterinburg male population.
These results are in agreement with the well-established fact that men have higher rates than women for most lymphoid neoplasm subtypes.Citation5 These results are significantly lower than in other regions; for example, sex-adjusted CLL prevalence in the Republic of Bashkortostan in the years 1999–2008 was 29.22 and 18.01 per 100 000 persons for men and women, respectively.Citation9
Cumulative 5-year (2009–2013) age-adjusted prevalence of CLL in the adult population
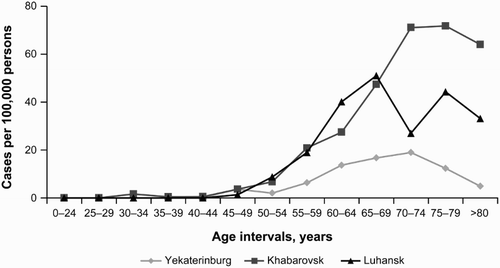
The highest mean cumulative CLL prevalence was recorded in Khabarovsk: in the age groups 70–74 years old – 71.30 (95% CI, 40.26–116.82), and 75–79 years old – 71.95 (95% CI, 32.56–137.50), per 100 000 persons in the population for both sexes. In the age groups <65 and ≥65 years old, the obtained values for the cumulative prevalence were 4.59 (95% CI, 2.94–6.83) and 63.03 (95% CI, 45.19–85.56), respectively (Fig. ).
The lowest mean cumulative CLL prevalence was recorded in Yekaterinburg. For comparison, in the age groups 70–74 and 75–79 years old, cumulative CLL prevalence was only 19.00 (95% CI, 8.40–36.85) and 12.34 (95% CI, 4.23–27.95) per 100 000 persons for both sexes, respectively, which is 3.75 and 5.83 times lower, respectively, than the values recorded in the same age groups in Khabarovsk. In the age groups <65 and ≥65 years old, the obtained values for the cumulative prevalence were 1.89 (95% CI, 1.20–2.84) and 13.39 (95% CI, 8.49–20.08), respectively.
The highest values for Luhansk, Ukraine, were recorded in the 65–69 years and 75–79 years age intervals: 51.18 (95% CI, 21.52–102.52) and 44.36 (95% CI, 13.98–105.21) per 100 000 persons for both sexes. In the <65 and ≥65 years age group, the obtained values for the cumulative prevalence were 5.58 (95% CI, 3.43–8.59) and 36.96 (95% CI, 23.53–55.26), respectively.
Conclusions
The results of this study show diverse CLL incidence and prevalence patterns in the adult population of the Russian Federation and Ukraine. A more comprehensive study with large region involvement is required to provide a more precise description of the incidence and prevalence of CLL in other regions and selected countries. The authors also propose initiating a national CLL register in the Russian Federation and Ukraine.
Results for Kazakhstan were omitted due to early withdrawal of this site from the study. Only approximately 80% of the required data for Luhansk were collected and processed due to the evolving political and social instability in the region, which should be considered during analysis of the study results.
The recorded cumulative 5-year (2009–2013) incidence of CLL in the adult population during the study ranged from 1.46 per 100 000 persons (95% CI, 0.85–2.34) in Yekaterinburg, Russia, to 4.32 per 100 000 persons (95% CI, 2.67–6.62) and 4.34 per 100 000 persons (95% CI, 2.48–7.04) in Khabarovsk, Russia, and Luhansk, Ukraine, respectively.
In 2013, the lowest prevalence of CLL was also recorded in Yekaterinburg: 7.11 per 100 000 persons (95% CI, 5.67–8.81). This was about 3.1 times lower than in both Khabarovsk (21.88 per 100 000 persons; 95% CI, 17.93–26.44) and Luhansk (21.92 per 100 000 persons; 95% CI, 17.38–27.28).
Various hypotheses exist as to why there is a discrepancy in CLL reporting across different regions. CLL mainly affects elderly patients who are less likely to undergo a thorough diagnostic assessment than younger patients. Another possible explanation is ‘excessive’ diagnostic activity in the USA, which could inflate incidence, especially in elderly patients in whom CLL is relatively common. Differences in the results of this study between cities with populations from 500 000 to over one million people could be related to differences in regional healthcare (mentioned above) that might lead to missed CLL cases.
Disclaimer statement
Contributors All authors took an active role in this study. Authors participated in critical revisions of the manuscript and have approved the article for publication. The authors contributed to the manuscript review, applying their clinical, epidemiology, and study design expertise.
Funding This study was sponsored and funded by GlaxoSmithKline.
Conflicts of interest The authors A.V., J.M., A.M., and C.M. are employed by GlaxoSmithKline. A.V. and C.M. own shares in GlaxoSmithKline. E.P. and M.H.S.P. are former employees of GlaxoSmithKline and E.P. is now employed by Novartis Pharmaceuticals, he also owns shares in Novartis Pharmaceuticals. A.L., V.M., and B.R. received investigator grants from GlaxoSmithKline for their involvement in the study.
Ethics approval None.
Acknowledgments
We thank all investigators for their contribution to the study. Ofatumumab is an asset of Novartis AG as of 2 March 2015. The sponsor was involved in the study design, collection, analysis, and interpretation of the data, in the writing of the report and in the decision to submit the article for publication.
References
- Wierda WG, Keating MJ, O'Brien S. Chronic lymphocytic leukemias. In: DeVita VT, Hellman S, Rosenberg SA, (eds.) Cancer principles & practice of oncology. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 2278–92.
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. Available from: http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER website.
- Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010;116(19):3724–34. doi: 10.1182/blood-2010-05-282632
- Watson L, Wyld P, Catovsky D. Disease burden of chronic lymphocytic leukaemia within the European Union. Eur J Haematol. 2008;81(4):253–8. doi: 10.1111/j.1600-0609.2008.01114.x
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107(1):265–76. doi: 10.1182/blood-2005-06-2508
- Davydov MI, Aksel EM. Statistics of malignant diseases in Russia and CIS countries in 2012. Moscow: NN Blokhin Russian Cancer Research Center RAM; 2014.
- Glusman DF, Sklyarenko LM, Nadgornaya VA, Koval SV. Chronic lympholeucosis; modern knowledge about etiology, pathogenesis, classification and diagnosis of the disease. Ukraine: Zdorovie; 2009. p. 38–40.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood 2009;111(12):5446–56. doi: 10.1182/blood-2007-06-093906
- Bakirov BA, Varshavskiy AV, Bessmeltsev SS. The dynamics of hemoblastosis morbidity in the republic of Bashkortostan (1962–2008). Hematology 2011;12(43):511–21.
- Panovská A, Doubek M, Brychtová Y, Mayer J. Chronic lymphocytic leukemia and focusing on epidemiology and management in everyday hematologic practice: recent data from the Czech Leukemia Study Group for Life (CELL). Clin Lymphoma Myeloma Leuk 2010;10(4):297–300. doi: 10.3816/CLML.2010.n.061
- Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;110(2):695–708. doi: 10.1182/blood-2006-11-051672
- Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858
- Park HJ, Park EH, Jung KW, Kong HJ, Won YJ, Lee JY, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012;47(1):28–38. doi: 10.5045/kjh.2012.47.1.28
- Mak V, Ip D, Mang O, Dalal C, Huang S, Gerrie A, et al. Preservation of lower incidence of chronic lymphocytic leukemia in Chinese residents in British Columbia: a 26-year survey from 1983 to 2008. Leuk Lymphoma 2014;55(4):824–7. doi: 10.3109/10428194.2013.827785
- Ruchlemer R, Polliack A. Geography, ethnicity and “roots” in chronic lymphocytic leukemia. Leuk Lymphoma 2013;54(6):1142–50. doi: 10.3109/10428194.2012.740670
- Kaprina AD, Starinskogo VV, Petrovoi GV, (pod red.). Zlokachestvennye novoobrazovaniia v Rossii v 2013 godu (zabolevaemost’ i smertnost’) - M.: MNIOI im. P.A. Gertsena - filial FGBU «FMITS im. P.A. Gertsena» Min-zdrava Rossii. - 2015. - ill. - 250 s. ISBN 978-5-85502-205-6.
- Seftel MD, Demers AA, Banerji V, Gibson SB, Morales C, Musto G, et al. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33(11):1463–8. doi: 10.1016/j.leukres.2009.06.013