Abstract
Objectives: Complex and multiple mechanisms are involved in the etiology of Hepatitis C virus-associated immune thrombocytopenia (HCV-ITP). Many hematopoietic growth factors affect the thrombopoiesis. The aim of this study was to clarify the interaction of the thrombopoietic factors in patients with HCV-ITP.
Methods: We selected 33 patients with HCV-ITP and 17 normal individuals. We compare serum interleukin (IL)-3, IL-6, IL-11, thrombopoietin (Tpo), stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor-α (TNFα), and spleen size between these two groups.
Results: Our study shows that Tpo, IL-6, and TNFα significantly increased in patients with HCV-ITP compared to the normal population (Tpo:122.577 vs. 40.602; IL-6: 2.175 vs. 0.943; TNFα: 2.460 vs. 1.322). IL-11 was significantly lower in the HCV-ITP group (10.829 vs. 15.042). HCV-ITP patients had a higher spleen index (21.121 vs 13.498, P = 0.003). According to regression analysis and multiple linear regression analysis, only IL-11 had a significantly positive correlation with platelet count, while TNFα showed a negative correlation.
Discussions: Tpo and IL-6 increased in patients with HCV-ITP, suggesting a positive feedback of low platelet count. TNFα-associated immune response is suspected to have an impact on low platelet count. IL-11 is assumed to directly affect thrombopoiesis.
Conclusions: This study is the most comprehensive study to evaluate the interaction between platelet count and the important thrombopoetic factors in patients with HCV-ITP. The thrombopoietic factors clearly play an important role in HCV-ITP.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by the immunologic destruction of normal platelets. ITP is classified into primary and secondary ITP, with primary ITP being induced by an unknown stimulus, which is diagnosed by exclusion, and secondary causes including autoimmune diseases, viral or bacterial infections, or certain drug exposure.Citation1 The most prevalent viral infection in ITP is hepatitis C virus (HCV) infection, which has been reported to range from 10 to 36% in different demographics.Citation2 Further, thrombocytopenia is frequently seen in chronic hepatitis C, with thrombocytopenia rates often being reported at 24% or more.Citation3 According to the American society of hematology 2011 guideline, it is named hepatitis C virus-associated immune thrombocytopenia (HCV-ITP).Citation1
HCV-ITP is assumed to be a distinct entity from primary ITP. The patients with HCV-ITP tend to be older and equally distributed between both genders in comparison with the female predominance in the patients with primary ITP. Severe thrombocytopenia with a platelet count of less than 10 000/uL is also less frequent in HCV-ITP.Citation4
Platelet count is dependent on the balance between platelet destruction and production. Multiple mechanisms have been reported to be related to hepatitis C infection and thrombocytopenia. Some factors that have been studied include splenic platelet sequestration, anti-platelet antibody formation, bone marrow suppression and the interaction of hematopoietic factors.Citation5–Citation7 Splenomegaly has been well-documented to increase the pooling of platelets in the spleen and the destruction of platelets.Citation8 Although splenomegaly is a significant adverse risk for low platelet count in chronic hepatitis C patients,Citation9 in clinical observation, many patients with HCV-ITP have normal-sized spleen but low platelet count; on the other hand, one-third of HCV-ITP patients have splenomegaly but normal platelet count.Citation10 In addition, many studies have tried to clarify the correlation between growth factors and platelet count. Because thrombopoiesis depends on the bone marrow microenvironment, which is composed of cellular and extracellular components, many hematopoietic growth factors affect the growth and maturation of megakaryocytes, including interleukin (IL)-3, IL-6, and IL-11, granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), and thrombopoietin (Tpo).Citation11 However, the role of Tpo in ITP has been controversial. Some studies have reported that Tpo decreased with severity of liver cirrhosis and was correlated with degree of thrombocytopenia. But others found no association between Tpo level and platelet count.Citation12 In clinical practice, Tpo-mimetic agents have been proved beneficial in treating HCV-ITPCitation13 and IL-11 has also been shown effective in increasing platelet count in patients with HCV-ITPCitation14 and in patients with thrombocytopenia after chemotherapy.Citation15 However, data are lacking on the changes of these critical megakaryopoietic growth factors in HCV-ITP. Until now, it remains unclear how these factors work together to affect platelet count in HCV-ITP. This study aims, therefore, to clarify the interaction of these main growth factors in thrombopoiesis in HCV-ITP.
Materials and methods
Patients
We studied patients with chronic hepatitis C and thrombocytopenia (<100 × 109/L) from December 2012 to November 2014. The diagnostic criteria were according to previous published guidelines.Citation1 Patients were aged over 18 years. Patients with severe liver cirrhosis, such as Child-Pugh classification B or C, concomitant chronic hepatitis B infection, alcohol abuse, autoimmune liver disease, hepatocellar carcinom, or other malignancies under chemotherapy, were excluded. Those who took medication known to result in thrombocytopenia in the proceeding 6 months were also excluded. None of the patients had been diagnosed with acute illness, including infection, bleeding, arterial, or venous thrombosis one month before enrollment. In addition, we also selected a healthy population with normal platelet count and without HCV infection as the control group. This study was approved by the institutional review board at the Chang Gung Memorial Hospital in accordance with the Declaration of Helsinki. All enrolled patients completed and provided their written informed consent for this study.
Clinical parameters
We checked the complete hemogram including white blood cell (WBC), hemoglobin (Hb), and platelet count. Baseline liver function tests, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (Alkp), albumin (Alb), and total bilirubin, were also collected. The patients with HCV-ITP were examined for the cirrhotic status by using the Child-Pugh classification, which included albumin, prothrombin time, total bilirubin, and abdominal ultrasonography. Spleen index was calculated as the length of the short axis multiplied by the length of the long axis, which crosses over the hilar with the short axis at a right angle. The sample of the control group (healthy volunteers without underlying liver disease) was checked as study group.
Cytokine analysis
Serum of patient and control group was collected into heparinized or EDTA containing blood and stored at −80°C until assays were performed. Cytokines were measured by means of an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions, including Il-3 (Human IL-3 Quantikine ELISA Kit), IL-6 (Human IL-6 Quantikine HS ELISA Kit), IL-11 (Human IL-11 Quantikine ELISA Kit), thrombopoietin (Tpo) (Human Thrombopoietin Quantikine ELISA Kit), Stem cell factor (SCF) (Human SCF Quantikine ELISA Kit), granulocyte-macrophage colony-stimulating factor (GM-CSF) (Human GM-CSF Quantikine ELISA Kit), and tumour necrosis factor-α (TNFα) (Human TNF-alpha Quantikine HS ELISA). These seven factors were all manipulated by R&D Systems, Inc. (Minneapolis, MN, USA). The principle of the above ELISA studies was to use antibodies raised against the receptor binding portion of the factors as the captured antibodies. These captured antibodies were all coated in 96-well EIA plates. Then, samples and the recombinant human cytokines, used as standard, were added. Signal antibodies using rabbit anti-cytokine conjugate were added after the uncaptured proteins were washed. TMB-peroxidase substrate and peroxidase solution were added for signal production and read for OD level. The detection range of the assay was correlated to the linear portion of the standard curve.
Statistical analysis
Student's two-tailed paired t-test was applied to compare the difference of serum cytokines between the HCV-ITP group and the healthy individuals. All values were expressed as mean and standard deviations. For analysis of statistical significance between the two groups, Student's two-tailed paired t-test was used, and P < 0.05 was considered significant. Correlation between two variables was evaluated using Spearman's coefficient of correlation method. Regression coefficients, R2, P-values were used to assess goodness-of-fit between cytokines and platelet count in the regression models. The level of significance was set at 0.05 for all values. All analyses were performed using the Statistical Package of Social Sciences software, version 19.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
A total of 33 patients with HCV-ITP were included in the study group (Table ). Eighteen of these 33 patients had chronic hepatitis, and the remaining 15 patients had early liver cirrhosis, Child-Pugh A. There were 17 normal control volunteers enrolled. There were no significant differences of age and gender between these two groups. The patients in the study group were female predominant with 21 women versus 12 men, but there was no significant gender difference between these two groups (P = 0.456). The average platelet count in the study group was 60.09 × 109/L, with a range of 5–98 × 109/L. The platelet count in the control group was 220.18 × 109/L, with a range of 113–353 × 109/L. The baseline white blood cell count and hemoglobin levels were similar in both groups. AST, ALT, and Alkp levels were significantly higher in the study group compared to the controls (mean level of AST 59.54 U/L vs. 18.75 U/L in the control, P < 0.001; ALT 66.53 U/L vs. 19.62 U/L in the control, P < 0.001; Alkp 96.78 U/L vs. 50.5 U/L in the control, P < 0.001). Serum albumin and total bilirubin levels were similar in both groups. Compared to the control group, patients with HCV-ITP had a significantly larger spleen index (21.121 vs. 13.498, P = 0.003), with 18 of the 33 patients having a spleen index larger than 20.
Table 1 Baseline characteristics of the patients with HCV-ITP and control group
Cytokine profile tests by ELISA
We checked seven cytokines associated with thrombopoiesis (Fig. and Table ). Among these cytokines, mean IL-11 level was significantly lower in the study group (10.829 pg/mL vs. 15.042 pg/mL in the control group, P = 0.049). In contrast, the mean level of Tpo, IL-6, and TNFα were significantly higher in the study group (Tpo:122.577 pg/mL vs. 40.602 pg/mL in the control group, P = 0.004; IL-6: 2.175 pg/mL vs. 0.943 pg/mL in the control group, P = 0.002; TNFα: 2.460 pg/mL vs. 1.322 pg/mL in the control group, P = 0.003, respectively). There was a trend of lower IL-3 levels in the HCV-ITP group (6.679 pg/mL vs. 8.340 pg/mL) compared to the normal controls, but it did not reach the level of statistical significance (P = 0.095). There were no significant differences in SCF and GM-CSF levels between the HCV-ITP and control groups.
Figure 1 Box plot of seven cytokines in HCV-ITP group and control group. (A) Interleukin (IL)-3, (B) IL-6, (C) IL-11, (D) Thrombopoietin (TPO), (E) Stem cell factor (SCF), (F) Granulocyte-macrophage colony-stimulating factor (GM-CSF), and (G) Tumour necrosis factor-α (TNFα). These cytokines were measured by enzyme-linked immunosorbent assay (ELISA) at least two independent exams according to the manufacturer's instructions.
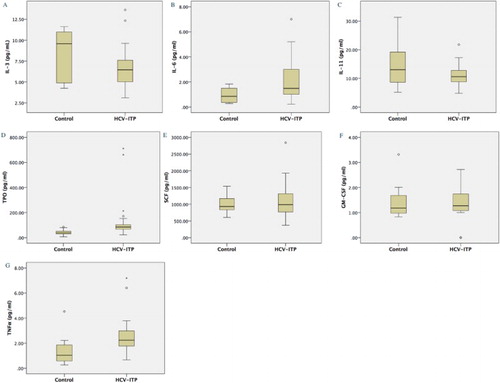
Table 2 Serum levels of thrombopoietic factors in study and control groups
In the linear regression analysis shown in Table , IL-3 and IL-11 showed a significant positive correlation with platelet count, R2 = 0.146 and 0.125, P = 0.015 and 0.012, respectively. On the other hand, TNFα showed a significant inverse correlation with platelet count (R2 = 0.144, P = 0.006). Tpo and IL6 were inversely correlated to platelet count, but it did not reach statistical significance (P = 0.094 and 0.051, respectively). Spleen index was also inversely correlated to platelet count, but also non-significantly so (R2 = 0.056, P = 0.148). In the multivariant regression analysis, we lumped the three factors of IL-3, IL-11, and TNFα with a significant impact on platelet count in a linear regression. IL-11 and TNFα still significantly correlated with platelet count (Table and Fig. ). Whereas the IL-11 level was significantly positively correlated to platelet count (P = 0.009), TNFα level negatively correlated with platelet count (P = 0.013).
Figure 2 Scatter plot and linear regression line of (A) Interleukin (IL)-11 and (B) Tumour necrosis factor-α (TNFα). These two thrombopoietic factors had significant correlation with platelet count. IL-11 has significantly positive correlated to platelet count, while TNFα has significantly inverse correlation.
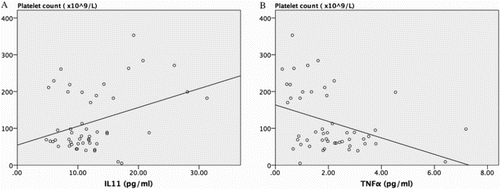
Table 3 Linear regression analysis of thrombopoietic factors and spleen index
Discussion
Immune thrombocytopenia is a highly heterogeneous hematological disorder. Some experts even suggest there are different characteristics between patients in Asia-Pacific region and western countries.Citation16 These also mean there are multiple complex mechanisms of chronic immune thrombocytopenia. One of the most important and studied thrombopoietic factors is Tpo. Tpo is produced and secreted by liver cells. However, the level of production in patients with thrombocytopenia has been a controversial issue. A somewhat larger number of studies have concluded that Tpo inappropriately increases in chronic ITP.Citation17 In chronic hepatitis patients, it decreases as liver disease progresses. But the role of Tpo in HCV-ITP patients has been controversial.Citation18,Citation19 In most studies with result of decreased Tpo levels in HCV-ITP patients compared to normal individuals, the enrolled study populations were always patients with variant status of liver cirrhosis.Citation18 However, to avoid certain confounding factors, we selected patients with chronic hepatitis C or early cirrhosis. Studies with patient population as our design have shown that Tpo still increases in patients with HCV-ITP compared to the normal population though the number of patients was relatively small.Citation19 Our study results echo this finding. This evidence suggests a feedback mechanism of thrombocytopenia in HCV-ITP patients as previous review article had mentioned.Citation7 Only recently has the mechanism of increasing Tpo been clarified as form of thrombocytopenia feedback.Citation20 A previous study found that immature platelet fraction (IPF) increased in patients with HCV-ITP,Citation9 which means that the lifespan of platelets decreased while the desialylated platelet count possibly increased. According to research on the Ashwell–Morell receptor (AMR) and associated activation of JAK2-STAT3 signaling, the binding of desialylated platelets to AMR drives hepatocytes to produce Tpo. However, the level of increase is relatively lower in HCV-ITP patients than in those with primary immune thrombocytopenia. Therefore, this explains why Tpo-mimetic has proven effective in treatment of HCV-ITP patients.Citation13
Serum IL-6 has been assumed to be an indicator of inflammation, insofar as mesenchymal cells, endothelial cells, and macrophages are the main producer of IL-6.Citation21 The level of IL6 significantly elevated in patients in our study with chronic hepatitis C compared to the normal individuals. Although in vivo and in vitro studies have reported that IL-6 increased platelet count, the impact of IL-6 in thrombopoiesis is indirect, with IL-6 stimulating thrombopoiesis through the expression of Tpo.Citation22,Citation23 Like the binding of AMR and desialylated platelet and downstream JAK2-STAT3 signaling pathway, the binding of IL-6 to its hepatic receptor drives JAK1-STAT3 to cascade and induce Tpo mRNA expression, thus increasing serum Tpo level.Citation24 Chronic inflammation induced by hepatitis C is one of the etiologies of increased IL-6 in HCV-ITP. However, in primary ITP patients without hepatitis-induced inflammation, IL-6 levels have also been reported to rise compared to those in the normal population.Citation25 It is possible that there is an unknown feedback mechanism other than inflammation in thrombocytopenia that follows from increased IL-6.
Interleukin-11 plays an important role in megakaryocytopoiesis and platelet production. In vitro, IL-11 is essential for the expansion and maturation of megakaryocytes when culturing stem cell or cord blood cell,Citation23 whereas in vivo, it has been used in the treatment of thrombocytopenia related to chemotherapy.Citation26 IL-11 also has been proven to be effective in the treatment of thrombocytopenia in patients with chronic hepatitis C by increasing platelet production.Citation14 However, the interaction of IL-11 in patients with thrombocytopenia still requires clarification. Until now, there has only been one study suggesting that IL-11 levels do not change in severe cirrhotic patients in a comparison of pre- and post-liver transplantation and their improvement of thrombocytopenia.Citation27 However, the population of that study was severe cirrhotic patients receiving liver transplantation. In our study, IL-11 significantly decreased in HCV-ITP patients. The enrolled patients in this study were those with chronic hepatitis and early cirrhosis with Child classification A, and they were compared to a normal population. This is the first study suggesting a decreased level of IL-11 in HCV-ITP and a correlation with platelet production. This can therefore explain directly why an IL-11 supplement is effective in HCV-ITP.
Tpo and IL-11 are two of the most important megakaryopoietic growth factors. We can see from our study that the reaction and mechanism of these two factors are different in the thrombocytopenia of patients with chronic hepatitis C infection. A previous study similarly identified different mechanisms for these two factors,Citation28 with an increase of Tpo being suggested as a positive feedback of an increased number of aged desialylated platelet and increased IL-6. On the other hand, IL-11 is deficient in HCV-ITP. As is commonly known, IL-11 is produced by many types of cells especially WBC, whereas Tpo is exclusively produced by liver cells. The gene of IL-11 is located on chromosome 19q13.3–13.4.Citation29 As previous studies have pointed out about the mechanism of hepatocellular carcinoma induced by HCV, HCV will induce the chromosomal abnormality of 19q.Citation30 The core protein of the hepatitis C virus induces the methylation of many genes or promoters, and 19q13.3–13.4 is one of the targeted areas.Citation31 Methylation of 19q13.3–13.4 may inactivate the IL-11 gene and reduce the production of IL-11 to decrease the platelet count. Further study is therefore warranted to clarify the relationship between HCV and the expression of IL11.
In the medical practice of anti-TNFα treatment in patients with rheumatoid arthritis, hematological complication with thrombocytopenia has occasionally been reported.Citation32 For this reason, it was assumed that TNFα should play a role in the thrombopoiesis and that the suppression of TNFα would induce thrombocytopenia. However, many studies have produced conflicting results about anti-TNFα treatment and have revealed that TNFα will induce thrombocytopenia.Citation33 Those studies and clinical observations indicate that the reaction between platelet count and level of TNFα is complex. In our study, TNFα significantly increased in patients with HCV-ITP, which suggests that there should be an interaction between platelet and TNFα. Our results further supported the inverse correlation between platelet count and level of TNFα. As previous studies have concluded, TNFα suppresses platelet count via Treg depression and autoimmune reaction rather than directly acting on thrombopoiesis.Citation34 The association between TNFα and autoimmune reaction such as anti-platelet autoantibody needs further study to clarify.
In addition to the effects of cytokines on megakaryopoiesis, spleen size has been always discussed as indicator of the destruction of platelets. In our study, all patients with HCV-ITP had early liver disease with chronic hepatitis or Child-Pugh A liver cirrhosis, but the spleen index significantly increased and was larger than that of the normal population. The mean index was about 21.12, which marks a borderline increase as most guidelines reference the level of 20 as boundary. Sequestration of platelet by an enlarged spleen supports this finding. However, in a regression study, no significant linear correlation was observed between spleen size and platelet count, which is consistent with previous studies.Citation9 In our study group, because 15 of the 33 patients had a spleen index less than 20, the effect of sequestration by spleen probably played a role in the thrombocytopenia in HCV-ITP but cannot completely explain the whole story. Another possible etiology, such as cytokine effects, is likely to also play a role in the complex mechanism.
There were some limitations in this study. First, the case number is limited, and a larger series is needed to confirm the above data. To find the etiology of thrombocytopenia in patients with HCV, we focused on the population with chronic hepatitis or early cirrhosis. However, the number of total 33 patients with HCV-ITP was larger than most other studies; thereby the case number has been always the main issue when fitting the criteria of thrombocytopenia by using a platelet count less than 100 × 109/L and excluding the confounding factor of severe cirrhosis. Second, in our study, we selected a healthy normal population as the control group as most of previous studies because the abnormal levels of cytokines should be referred and compared to normal population. And the normal range of certain thrombopoietic factors have not been standardized. However, some question need to be answered by comparing non-thrombocytopenia in patients with chronic hepatitis C.
In conclusion, we have clarified the interaction of critical megakaryopoietic factors in patients with HCV-ITP. In our study, Tpo, IL6, and TNFα significantly increased in HCV-ITP patients compared to the normal controls. Only TNFα significantly inversely correlated with platelet count. In contrast, IL-3 and IL-11 were positively correlated to platelet count but only IL-11 level significantly decreased in patients with HCV-ITP. These thrombopoietic factors clearly play an important role in thrombocytopenia in patients with HCV-ITP. Splenomegaly as platelet sequestration was also a factor. The mechanism of thrombocytopenia in patients with HCV infection is complex requires further investigation.
Disclaimer statement
Contributors CEH and CCC conceptualized and wrote the manuscript. CEH, YYC, JJC, FCK, KDL, CHL, CHS, and CCC collected patient data, provided patient care, and wrote the manuscript. CHS performed the abdominal echo. CEH and JL did cytokine analysis.
Funding This study was supported by Chang Gung Memorial Hospital grants to Cih-En Huang (CMRPG6B0301) and to Chih-Cheng Chen (CORPG6B0373).
Conflicts of interest All authors report no declarations of interest.
Ethics approval This study was approved by the institutional review board at the Chang Gung Memorial Hospital in accordance with the Declaration of Helsinki.
References
- Neunert C, Lim W, Crowther M, Cohen A, Solberg Jr L, Crowther MA, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984
- Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1275–97. doi: 10.1016/j.hoc.2009.08.009
- Louie KS, Micallef JM, Pimenta JM, Forssen UM. Prevalence of thrombocytopenia among patients with chronic hepatitis C: a systematic review. J Viral Hepat. 2011;18(1):1–7. doi: 10.1111/j.1365-2893.2010.01366.x
- Rajan SK, Espina BM, Liebman HA. Hepatitis C virus-related thrombocytopenia: clinical and laboratory characteristics compared with chronic immune thrombocytopenic purpura. Br J Haematol. 2005;129(6):818–24. doi: 10.1111/j.1365-2141.2005.05542.x
- Weksler BB. Review article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther. 2007;26(Suppl 1):13–9. doi: 10.1111/j.1365-2036.2007.03512.x
- Afdhal NH, McHutchison JG. Review article: pharmacological approaches for the treatment of thrombocytopenia in patients with chronic liver disease and hepatitis C infection. Aliment Pharmacol Ther. 2007;26(Suppl 1):29–39. doi: 10.1111/j.1365-2036.2007.03511.x
- Tillmann HL, McHutchison JG. Use of thrombopoietic agents for the thrombocytopenia of liver disease. Semin Hematol. 2010;47(3):266–73. doi: 10.1053/j.seminhematol.2010.04.003
- Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45(5):645–57. doi: 10.1172/JCI105380
- Zucker ML, Hagedorn CH, Murphy CA, Stanley S, Reid KJ, Skikne BS. Mechanism of thrombocytopenia in chronic hepatitis C as evaluated by the immature platelet fraction. Int J Lab Hematol. 2012;34(5):525–32. doi: 10.1111/j.1751-553X.2012.01429.x
- Adinolfi LE, Giordano MG, Andreana A, Tripodi MF, Utili R, Cesaro G, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol. 2001;113(3):590–5. doi: 10.1046/j.1365-2141.2001.02824.x
- Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009:147–52.
- Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23(8):1055–65. doi: 10.1111/j.1365-2036.2006.02889.x
- Maan R, Veldt BJ, Janssen HL. Eltrombopag for thrombocytopenic patients with chronic HCV infection. Gastroenterol. 2014;147(1):254–5. doi: 10.1053/j.gastro.2014.01.070
- Lawitz EJ, Hepburn MJ, Casey TJ. A pilot study of interleukin-11 in subjects with chronic hepatitis C and advanced liver disease nonresponsive to antiviral therapy. Am J Gastroenterol. 2004;99(12):2359–64. doi: 10.1111/j.1572-0241.2004.40047.x
- Cantor SB, Elting LS, Hudson Jr DV, Rubenstein EB. Pharmacoeconomic analysis of oprelvekin (recombinant human interleukin-11) for secondary prophylaxis of thrombocytopenia in solid tumor patients receiving chemotherapy. Cancer 2003;97(12):3099–106. doi: 10.1002/cncr.11447
- Lee LH, Caguioa P, Chin NS, Chiou TJ, Lee JW, Miyakawa Y, et al. Chronic adult primary immune thrombocytopenia (ITP) in the Asia-Pacific region. Int J Hematol. 2011;94(2):142–9. doi: 10.1007/s12185-011-0894-8
- Kuter DJ, Gernsheimer TB. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1193–211. doi: 10.1016/j.hoc.2009.09.001
- Pradella P, Bonetto S, Turchetto S, Uxa L, Comar C, Zorat F, et al. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54(5):894–900. doi: 10.1016/j.jhep.2010.08.018
- Aref S, Mabed M, Selim T, Goda T, Khafagy N. Thrombopoietin (TPO) levels in hepatic patients with thrombocytopenia. Hematology 2004;9(5–6):351–6. doi: 10.1080/10245330400010620
- Grozovsky R, Begonja AJ, Liu K, Visner G, Hartwig JH, Falet H, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015;21(1):47–54. doi: 10.1038/nm.3770
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/S0065-2776(08)60532-5
- Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood 2001;98(9):2720–5. doi: 10.1182/blood.V98.9.2720
- Lu H, Jiang T, Li R, Wang S, Zhang Q, Zhao S. Bone marrow stromal cells transduced with a thrombopoietin, interleukin-6, and interleukin-11 syncretic gene induce cord mononuclear cells to generate platelets in vitro. Transfusion 2015;55(1):176–86. doi: 10.1111/trf.12800
- Eulenfeld R, Dittrich A, Khouri C, Muller PJ, Mutze B, Wolf A, et al. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol. 2012;91(6–7):486–95. doi: 10.1016/j.ejcb.2011.09.010
- Kosar A, Haznedaroglu IC, Buyukasik Y, Ozcebe O, Kirazli S, Dundar S. Circulating thrombopoietin and interleukin-6 in newly diagnosed autoimmune versus aplastic thrombocytopenia. Haematologica 1998;83(11):1055–6.
- Tepler I, Elias L, Smith 2nd JW, Hussein M, Rosen G, Chang AY, et al. A randomized placebo-controlled trial of recombinant human interleukin-11 in cancer patients with severe thrombocytopenia due to chemotherapy. Blood 1996;87(9):3607–14.
- Peck-Radosavljevic M, Zacherl J, Wichlas M, Sims P, Meng YG, Panzer S, et al. Thrombopoietic cytokines and reversal of thrombocytopenia after liver transplantation. Eur J Gastroenterol Hepatol. 1999;11(2):151–6. doi: 10.1097/00042737-199902000-00015
- Chang M, Suen Y, Meng G, Buzby JS, Bussel J, Shen V, et al. Differential mechanisms in the regulation of endogenous levels of thrombopoietin and interleukin-11 during thrombocytopenia: insight into the regulation of platelet production. Blood 1996;88(9):3354–62.
- Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood 1997;89(11):3897–908.
- Sakakura C, Hagiwara A, Taniguchi H, Yamaguchi T, Yamagishi H, Takahashi T, et al. Chromosomal aberrations in human hepatocellular carcinomas associated with hepatitis C virus infection detected by comparative genomic hybridization. Br J Cancer. 1999;80(12):2034–9. doi: 10.1038/sj.bjc.6690638
- Kondo Y, Kanai Y, Sakamoto M, Mizokami M, Ueda R, Hirohashi S. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis – A comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology 2000;32(5):970–9. doi: 10.1053/jhep.2000.19797
- Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, Van Assche G. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36(4):312–23. doi: 10.1111/j.1365-2036.2012.05189.x
- Piguet PF, Vesin C, Da Kan C. Activation of platelet caspases by TNF and its consequences for kinetics. Cytokine 2002;18(4):222–30. doi: 10.1006/cyto.2002.0889
- Tawadrous GA, Aziz AA, Amin DG, Eldemery A, Mostafa MA. RANTES, TNF-α, oxidative stress, and hematological abnormalities in hepatitis C virus infection. J Invest Med. 2012;60(6):878–82. doi: 10.2310/JIM.0b013e318254519e
