Abstract
Objectives: Monoclonal anti-human blood group A (51A8) and B (63B6) antibody reagents were prepared using the serum-free technique. The aims of this research were to characterize the serum-free reagents and prove their reliabilities in routine use.
Methods: Experiments including antigen–antibody agglutination testing, stability testing, SDS–PAGE, protein and IgM quantification, flow cytometry, and variable domain sequencing were performed to characterize the anti-A (51A8) and anti-B (63B6) reagents. Over 12 000 samples were tested using these reagents as routine blood grouping reagents.
Results: Serum-free anti-A (51A8) and anti-B (63B6) reagents were stable in longitudinal and accelerated testing, and their high purity was shown in SDS–PAGE and IgM quantification. These reagents have high specificity to red blood cells in serologic agglutination testing and flow cytometric analysis. A1 and A2 subgroup antigens can be distinguished clearly by patterns of flow cytometric histograms. No discrepancy was found in clinical trials of 12 000 samples.
Discussion: To reduce the risk of being affected by any animal additives, a serum-free culture system was applied to get mass-production of monoclonal anti-A/B antibodies. The high specificity and the high purity of the reagents were verified by the lab experiments.
Conclusion: Lab research and clinical trial showed that serum-free monoclonal anti-A (51A8) and anti-B (63B6) reagents meet the requirements of routine blood grouping reagents. Moreover, these reagents featured ultra-high purity that is missing in other commercial counterparts, and therefore are recommended as more environment-friendly reagents.
Introduction
In 1981, Sacks and LennoxCitation1 reported the application of human monoclonal anti-B antibody stably secreted by hybridoma cells through hybridoma technology as a blood grouping reagent. Human A/B blood grouping reagents were replaced by monoclonal antibodies later. Lévy et al.Citation2 characterized the biological properties of murine anti-blood group A monoclonal antibody. The international standard for anti-A or anti-B monoclonal blood grouping reagents was further evaluated by Wittig et al.Citation3 and Thorpe et al.Citation4 in 2006. Today, anti-blood group A/B monoclonal antibodies are one of the most widely used blood grouping reagents. Although the specificity and the affinity of antibody–antigen reaction were recognized by the manufacturing enterprises or the laws as the golden standard to evaluate the quality of those applied antibodies, limited studies were conducted on the purity of the antibody or any potential factors that might be harmful to the environment or even threatening to the health of humans. Therefore, we developed environment-friendly anti-human blood group A/B monoclonal antibodies.
High-purity anti-A and anti-B monoclonal antibodies were obtained in the serum-free culture system and these antibodies were characterized by serological methods and molecular biology methods. So far, the evaluation of antigen–antibody agglutination reaction was qualitative or semi-quantitative. In 2003, Roback reported a newly developed flow cytometry (FC) technique to test for ABO group, D type, and the presence of red blood cell (RBC) alloantibodies. They found that FC testing methods, comparable in accuracy to standard column agglutination technology (CAT) and tube methods, allow rapid and cost-effective immunohematology testing of both patient and donor samples in an automated workstation format.Citation5,Citation6 In our research, the specificity of monoclonal antibodies to ABO RBCs was also tested by the FC method. Five independent medical institutions in China were chosen to test over 12 000 samples by our antibodies. It has been proved that those monoclonal antibodies developed in this study meet the requirements of blood grouping reagents.
Materials and methods
Preparation of monoclonal antibodies
Murine monoclonal anti-A and anti-B were obtained by the fusion of the mouse myeloma cell line SP2/0 with immune spleen cells. The immunogens used were type A1 or type B RBCs. The fusion process was followed by Guo's method,Citation7 and we introduced a new homemade V9 serum-free medium (Biosci, Shanghai, China) instead of standard RPMI-1640. No animal serum was used during the cell culture.
For mass-production of antibodies, hybridoma cells secreting anti-A or anti-B antibodies were first plated in T75 flasks (Corning, New York, USA) with V9 medium until it reaches 1.5 × 106/ml density. The cells were split to 2-l blue-cap glass bottles (Duran, Mainz, Germany) with 200 ml V9 medium, and then the bottles were moved to a shaker with the speed of 100 rpm at 37°C for continuing cultivation. Four hundred milliliters of fresh V9 medium was added to each bottle to reach 600 ml total medium after 2 days for continuing cultivation. Cell number counting and antibody titer of culture supernatant testing were performed in the following 8 days. When cultures gave optimum antibody titer, the cells were removed by centrifugation at 3000 rpm for 20 minutes, and the raw cell supernatants were filtered by 0.2-µm filter and used directly as monoclonal antibody reagents for following tests without concentration.
Cell number counting and antibody titer testing
Cell number counting
To check the cell viability, mix 20 µl of cell suspension with 20 µl of 0.4% trypan blue in PBS and calculate number of stained (died) cell to the total cell number in a hemocytometer.
Antibody titer testing
A series of doubling dilutions of the anti-A or anti-B reagents for testing were prepared in saline in a microtiter plate, then 3% RBC suspension was added to each well. The mixtures were incubated for 15–20 minutes at room temperature before reading the results.
Reagents for comparison
Several wildly used commercial anti-A and anti-B monoclonal antibody reagents including Merck-Millipore (Darmstadt, Germany) BIOSCOT Anti-A and Anti-B, Sanquin (Amsterdam, Netherland) Pelikloon Anti-A and Anti-B, Beijing Kinghawk (Beijing, China) Anti-A and Anti-B, and Shanghai Hemo-pharmaceutical & Biological Co., Ltd. (Shanghai, China) Anti-A and Anti-B were selected for comparison with our anti-A (51A8) and anti-B (63B6) reagents. These reagents were used following the user guide.
Antibody stability testing
Longitudinal testing
Anti-A and anti-B reagents were stored at 2–8°C for 2 years. Evaluation of the antibody reagents, including appearance, specificity, and titer, was performed on the 0, 6th, 12th, 18th, 20th month, respectively.
Accelerated degradation testing
Anti-A and anti-B reagents were stored at 37 or 43 °C for 3 weeks. Evaluation of the antibody reagents was performed on the 0, 3rd, 7th, 14th, 21st day, respectively. Freeze–thaw experiment for the reagents was cycled between −70 and 37°C for nine times. The antibody reagents were stored at each temperature for at least 1 hour for freezing or thawing completely.
Antibody purity testing
SDS–PAGE
Monoclonal antibody reagents were first mixed with 5× loading buffer (with 2% SDS and 5% 2-mercaptoethanol) and boiled at 100°C for 5 minutes. Samples were centrifuged at 12 000 rpm for 2 minutes before electrophoresis, then 5 µl supernatant of each sample was analyzed with a 15% gel.
Bradford protein quantification
Twenty microliters of antibody sample or bovine serum albumin (BSA) standard was added to immuno polystyrene module plates (Nunc, Roskilde, Denmark), then 200 µl Bradford reagent (TransGen Biotech, Beijing, China) was added to each well and incubated at room temperature for 5–10 minutes. Optical density was read at 595 nm in Multiskan FC Microplate Photometer (Thermo-Scientific, Waltham, USA).
ELISA IgM quantification
Immuno polystyrene module plates (Nunc, Roskilde, Denmark) were coated with 100 µl/well of 2 µg/ml anti-mouse IgM (Sigma-Aldrich, St. Louis, USA), then stored at 43°C for 1 h. Nonspecific binding sites were blocked with 1% BSA in PBS at 43°C for 30 minutes and the coated plates were washed by PBS containing 0.05% Tween-20 for three times. Hundred microliters of antibody sample or reference (mouse IgM, Sigma-Aldrich, St. Louis, USA) was added and incubated at 43°C for 1 hour. The plates were washed by PBS containing 0.05% Tween-20 for five times, then 100 µl goat anti-mouse IgM-Peroxidase (Sigma-Aldrich, St. Louis, USA) which was diluted 1:4000 in 1% BSA in PBS containing 0.05% Tween-20 was added. The reaction began when 75 µl 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Thermo-Scientific, Waltham, USA) was added, and stopped after 15 minutes with 75 µl TMB stop solution (Thermo-Scientific, Waltham, USA). Optical density was read at 450 nm in Multiskan FC Microplate Photometer (Thermo-Scientific, Waltham, USA).Citation8
Flow cytometric analysis
Three percent of RBC saline (Shanghai Hemo-pharmaceutical & Biological Co., Ltd, Shanghai, China) was washed twice with PBS. Twenty-five microliters of packed RBCs (about 80% RBCs) was suspended with 975 µl fixation buffer (0.01 M PBS with 0.05% glutaraldehyde and 0.04% formaldehyde) and incubated for 20 minutes at room temperature. Fixed RBCs were washed once with PBS (with 6% BSA), and washed twice with PBS (with 0.6% BSA), and then suspended with 640 µl PBS (with 0.6% BSA) to make a fresh 3% RBC saline suspension.Citation9
Flow cytometric experiment and data analysis was performed on the flow cytometer (BD FACSCanto II). Briefly, anti-A or anti-B antibody and anti-Mouse IgM (µ-chain specific)-FITC (Sigma Cat#F9259) were used as the first and second antibody, respectively. Twenty microliters of 3% fixed RBC saline suspension was incubated with 50 µl of first antibody for 30 minutes at room temperature, and washed once with 1 ml PBS (with 0.6% BSA), and then suspended with 50 µl PBS (with 0.6 BSA). Ten microliters of second antibody was added to the suspension and incubated for 30 minutes at room temperature. The treated RBCs were added 200 µl PBS (with 0.6% BSA) before flow cytometric analysis. The data analysis gate was set to include the viable part of the RBC population (set to P1), excluding deformed and aggregated cells.Citation10 The cells with FITC-derived fluorescence intensity from 102 to 105 were gated as P2 for counting positive rate.
Sequencing of variable domains (VH/VL)
Total cellular RNA was extracted from 5 × 106 hybridoma cells by using TRIzol (ShineGene, Shanghai, China) following the user guide. First-strand cDNA synthesis was performed with an oligo (dT) primer and M-MLV reverse transcriptase (Promega, Madison, USA). The primers used for the amplification of IgM variable domains are listed below: 5′-AAGGCCCAACCGGCCATGGCCCAGRTYCAGCTGGTGCAGTCTGG-3′ (VH-forward); 5′-CCGCGGCCGCGCCACCAGATTCTTATCAG-3′ (VH-reverse); 5′-AAGGCCCAACCGGCCGMCA TYCAGWTGACCCAGTCTCC-3′ (VL-forward); 5′-CCGCGGCCGCACACTCTCCCCTGTTGAAGCTCTT-3′ (VL-reverse).Citation2,Citation11 ‘Touchdown’ PCR was carried out by 11 cycles of denaturation at 94°C for 10 seconds, annealing at a temperature decreasing from 65 to 55°C over 30 seconds, and elongation at 68°C for 2 minutes and then followed by 30 cycles of denaturation at 94°C for 10 seconds, annealing at 55°C for 30 seconds, and elongation at 68°C for 2 minutes.Citation11,Citation12 PCR productions were analyzed by agarose gel electrophoresis and submitted for sequencing (Sangon Biotech, Shanghai).
Clinical trial
In cooperation with five independent medical institutions in China including Shanghai Xinhua Hospital, Shanghai Sixth People's Hospital, Guangzhou LiuHuaQiao Hospital, Ulanqab Blood Center (Inner Mongolia), and Dehong Dai and Jingpo Autonomous Prefecture Blood Center (Yunnan), monoclonal anti-A (51A8) and anti-B (63B6) reagents have been used to type over 12 000 blood samples including 842 samples of Chinese ethnic minorities. Peripheral blood samples from healthy blood donors, cancer patients, autoimmune patients, and other patients have been tested by our monoclonal antibody reagents. Hemolyzed samples, hyperlipidemic samples, and polluted samples were eliminated from the experiments. The ABO blood type of samples was determined by the antigen–antibody agglutination reaction (tube method or plate method).Citation13
Results
Cell growth and antibody producing
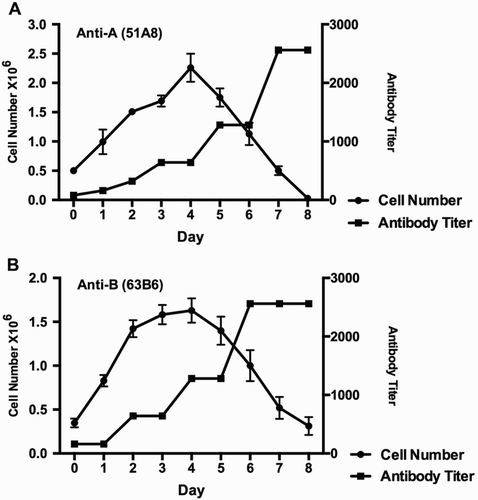
Among hundreds of hybridoma cell clones, two clones – 51A8 and 63B6 were picked. Both clones produce antibodies with best efficiency and have best mitotic stability (data not shown). The clones 51A8 and 63B6 stably secrete anti-A and anti-B monoclonal antibodies, respectively. The cell growth curve and the antibody titer of 51A8 and 63B6 during the producing process are as shown in Fig. . Exponential growth phase occurred at the beginning and the cell number reached maximum at day 4 and then reduced gradually till day 8. However, antibody productivity in the supernatant increased gradually until it reached plateau state at day 7 for anti-A (51A8) (Fig. A) or day 6 for anti-B (63B6) (Fig. B), suggesting that these hybridoma cells continually secrete antibody until they die.
Figure 1 Cell growth curve and antibody productivity. The counted cell number of clone 51A8 (A) and 68B6 (B) in 600 ml medium (indicated as solid circle) and measured anti-A (A) or anti-B (B) antibody titer (indicated as solid square) of the culture supernatants everyday were plotted. Bar represents standard deviation (SD) of three parallel samples.
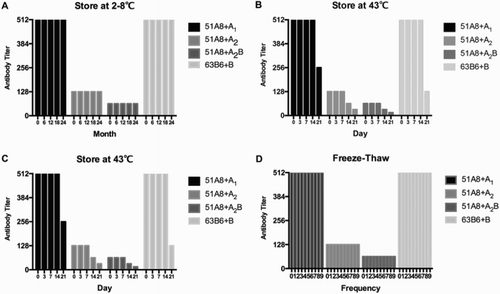
Antibody stability
The stability of our monoclonal anti-A/B antibody reagents were then tested for their possibility of meeting the requirement of routine use. All the stability tests referred to the Chinese Pharmacopoeia and followed the regulations of China Food and Drug Administration. A1, A2, A2B RBCs were used for anti-A titration, while B RBCs were used for anti-B titration. As the results shown in Fig. A and B, the reagents were stable at 2–8°C for 2 years and 37°C for 3 weeks, at least. While stored at 43°C, the anti-A reagents began to deteriorate after 1 week, while the anti-B reagents were stable for 2 weeks (Fig. C). Freeze–thaw cycling between −70 and 37°C for nine times did not reduce the titer of the reagents (Fig. D). Taken together, these results suggest the high stability of our monoclonal antibody reagents, which allows a wild range of applications in the future.
Antibody purity
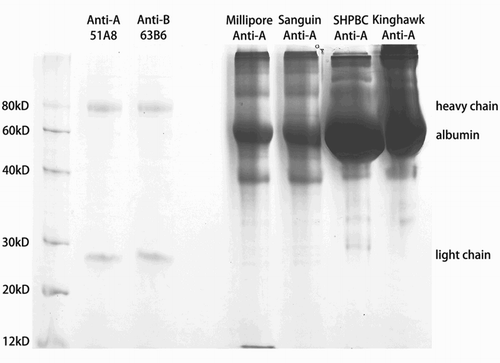
In this study, a homemade serum-free culture system was applied to obtain anti-A and anti-B antibody reagents excluding any animal supplements, such as bovine serum. The purity and concentration of our anti-A/B reagents were compared with other widely used commercial counterparts by protein quantification assays. Four commercial anti-A reagents were used as control in the SDS–PAGE method. The heavy chain and light chain of both anti-A (51A8) and anti-B (63B6) reagents were clearly shown on SDS–PAGE (Fig. ) with small amount of impurities, whereas the control anti-A reagents showed very weak or invisible IgM bands but full of impurities including a dense band of BSA. Because of high concentration of proteins, all the undiluted control anti-A reagents from other companies showed protein precipitation after boiling. Some of them did not precipitate after boiling, and also showed bands of heavy chain and light chain after 20 times dilution (data not shown).
Figure 3 SDS–PAGE analysis of anti-A (51A8) and anti-B (63B6) reagents and other commercial anti-A reagents.
Besides SDS–PAGE, we also analyzed the total protein concentration and the particular concentration of IgM in the mixture of our and other antibody reagents by Bradford assay and ELISA (Table ). Results showed that the antibody reagents produced by our serum-free technique have high IgM concentration but much lower total protein concentration compared to other commercial counterparts. The IgM/total protein concentration ratio is about 1.00 for our antibody reagents, but very low in others (Table ). Taken together, the concentration of total proteins and IgM measured by Bradford and ELISA, respectively, confirmed the high purity of our anti-A and anti-B reagents.
Table 1 Protein quantification of different antibody reagents
Antibody specificity
To test the specificity of our monoclonal anti-A and anti-B antibody reagents, we performed antigen–antibody agglutination reactions using different RBCs. For common antigens, anti-A (51A8) agglutinated groups A1, A2, A1B, and A2B RBCs; anti-B (63B6) agglutinated groups B, A1B, and A2B RBCs based on tube and plate methods (Table ). All these reactions gave strong agglutination and could be scored 12 points according to the system described by Marsh.Citation14 Our antibodies were also tested with rare subgroup antigens. Anti-A (51A8) and A3, anti-B (63B6) and ABx showed serologic mix-field reactions by tube and plate methods, which could be scored 6–8 points. Anti-B (63B6) had weaker reactivity with Bx RBCs, and most of the unagglutinated Bx RBCs formed a pellet at the bottom of the column in CAT experiments.Citation15,Citation16 Anti-A antibody did not react with B RBCs, the vice versa. Neither of the anti-A nor anti-B antibodies showed positive reaction with O RBCs.
Table 2 Agglutination score of anti-A (51A8) and anti-B (63B6) reagents reacted with different RBCs phenotype
As mentioned above, our monoclonal anti-A/B reagents are much purer than that of other four commercial reagents. Further investigation was made to compare our reagents with these counterparts in antibody titer. As shown in Table , our reagents have high antibody titer of 1:2560, which is as good as the reagents from Merck-Millipore and Sanquin, and much better than two others. The antibody titer is directly proportional to the concentration of IgM of the antibody reagents (Table ).
Table 3 Titer of anti-A and anti-B reagents from different manufacturers
For further analysis of the specificity of the reaction between ABO antigens and our antibody reagents, FC experiments were performed. A1, A2, A1B, A2B, B, and O RBCs were used to react with anti-A or anti-B antibody reagents, respectively. Figure shows the expression patterns with respect to the intensity of the FITC-derived fluorescence of RBCs of various representative phenotypes tested with anti-A (51A8) and anti-B (63B6). When tested with anti-A (51A8) antibody reagents, RBCs of types A1 and A1B were clearly positive and the value of P2 was 98.9 and 93.5%, respectively, forming sharp peaks. RBCs of types A2 and A2B got relatively lower P2 value of 88.7 and 92%, forming broad peaks. From two types of peak shapes and number of positive cells, we can clearly distinguish A1 and A2 antigens. When tested with anti-B (63B6) antibody reagents, A2B and B antigens showed similar positive rate and peak shape, but A1B antigen showed a uniform histogram with dual cell populations and its positive rate (81.3%) was lower than A2B (99.2%) and B (98%) antigens. Anti-A (51A8) showed negative results when tested with B and O antigens, and anti-B (63B6) showed negative results when tested with A and O antigens.
Figure 4 Histograms of RBCs of ABO antigens tested with anti-A or anti-B antibody reagents and FITC-labeled secondary antibodies. The X and Y-axis represent FITC-derived fluorescence and the number of cells, respectively.
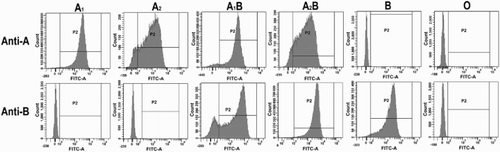
Figure 5 The comparison of amino acid sequences of the variable regions of anti-A (51A8) and anti-B (63B6) with that of other published counterpart.Citation17–Citation19 (A and B) VH and VL sequences of 51A8 are compared with those of AC12.Citation2 (C and D) VH and VL sequences of 63B6 are compared with those of BC1004.Citation20
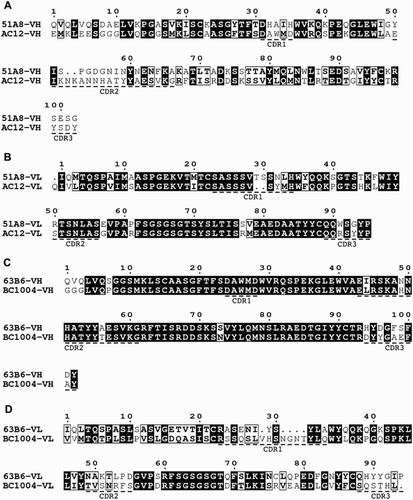
Sequencing of variable domains
To characterize the genes of our clones studied above, we sequenced the cDNA of the variable regions of clone 51A8 and 63B6 (Fig. ). The amino acid sequences of the variable regions of the heavy chain and the light chain of 51A8 were compared with that of another mouse monoclonal anti-human blood group A antibody (AC12).Citation2 51A8 and AC12 had similar VL sequences (identity = 79%) but much less similar VH sequences (identity = 42%). 63B6 was compared with another mouse hybridoma antibody to human blood group B (BC1004).Citation20 They had similar VH sequences (identity = 89%) but less similar VL sequences (identity = 49%). These results are contrary to Chen's work,Citation20 and suggest that both VH and VL sequences decide the specificity of antibodies.Citation21
Clinical trial
After lab experiments, monoclonal anti-A (51A8) and anti-B (63B6) reagents were tested as routine blood grouping reagents in five independent medical institutions in China. Total number of the blood samples is 12 056, and the agglutination results using anti-A (51A8) and anti-B (63B6) reagents are as shown in Table . There were no discrepancies between the ABO RBC groups of blood samples and the typing of our monoclonal antibody reagents. All the samples, including one sample of AsubB, were typed successfully, indeed proving the reagents’ reliability in routine use.
Table 4 Results of clinical trial of anti-A (51A8) and anti-B (63B6) reagents
Discussion
As one of the most widely used antibody reagents, commercial monoclonal anti-human blood group A/B reagents are the filtered supernatant of hybridoma cell culture and stored with preservatives. They meet the requirement of reacting with human RBCs, but the existence of animal proteins such as BSA is unavoidable. High-quality BSA is mainly dependent on importation in China. Chinese government imposed a ban on importation of bovine origin products, including bovine blood products, from the countries with a bovine spongiform encephalopathy risk status in 2001, because of the potential clinical risk factors. The production of products which require BSA was affected more or less in China for several years. Though the ban was lifted in 2014, future recurrence is unpredictable. Furthermore, the pharmacopoeia of some countries expressly allows the direct addition of mice ascites, which violates the ethics of animal experimentation. The prevalence of these diagnostic reagents may accumulate potential factors threatening human health. To reduce the risk of being affected by any animal additives, we applied an economical, efficient, and convenient culture system to get mass-production of monoclonal anti-A/B antibodies. The advantage of this system is that additional CO2 and O2 are unnecessary, and the culture medium is free of bovine serum and other additives like insulin and transferrin. The antibody production cycle is about 2 weeks, and highly pure antibody products can be obtained without any concentration or purification of cell culture supernatant.
The SDS–PAGE and IgM quantification show a lot of impurities in other standard commercial anti-A/B reagents. We analyzed the high-molecular-weight proteins (>80 kD) from the SDS–PAGE gel (Fig. ) by mass spectra, and the result showed the existence of actin, desmoplakin, serum albumin, and many other proteins (data not shown). These proteins could be brought in by cell disruption during the process of antibody preparation or by addition of bovine serum during cell culturing. By using serum-free culture medium and protein-free stabilizer, our antibody reagents contained much less extrinsic proteins as well as impurities from hybridoma cells without affecting the stability.
Classical serologic experiments can determine the blood group of antigens and test the titer of antibodies, but flow cytometric analysis can provide more particular data to show the specificity of antibodies.Citation22 Except for the positive rate of antigen–antibody reaction, our FC data showed different patterns of A1 and A2 antigens detected by our monoclonal anti-A/B reagents, which is similar to Hult's result.Citation10 The phenomenon could be related to the antigenic determinant of erythrocytes recognized by the antibodies,Citation23 or the number of the antigenic determinants of the A1 and A2 antigens.Citation24
Serologic experiments and flow cytometric analysis proved that anti-A (51A8) and anti-B (63B6) reagents react avidly with antigens of common blood phenotypes. Despite relatively weaker reaction with subgroup antigens, the reagents succeeded in grouping 12 000 blood samples in clinical trial (Table ), including one sample of AsubB. Mix-field and weak agglutinations suggest subgroup antigens in grouping experiments.
Taking the advantage of serum-free culture system, our monoclonal anti-A/B antibody reagents are more environment-friendly than the other commercial anti-A and anti-B blood grouping reagents. The specificity and stability of our anti-A (51A8) and anti-B (63B6) reagents at least meet all the requirements of routine use. The purity and safety of antibody-related products, such as diagnostic reagents and antibody drugs, could be improved a lot if preparation methods in this study were popularized, and it will be a useful application in the monoclonal antibody industry.
Disclaimer statements
Contributors Chenjie Zhou and Xuechao Gao analyzed the data and wrote the manuscript. Chenjie Zhou, Shixiang He, Xiaoling Gao, Jialin Zhuang, and Lirong Huang contributed to the experiments of the research. Hengchang Guo and Chenjie Zhou designed the research study.
Funding This work was supported by the Shanghai Science and Technology Committee under Grant 1302H193800.
Conflicts of interest The authors declare that they have no competing interests.
Ethics approval The clinical trial in this study was approved by the ethics committees of Shanghai Xinhua Hospital, Shanghai Sixth People's Hospital, and Guangzhou Liuhuaqiao Hospital.
Acknowledgments
We thank Professor Guangping Luo from Guangzhou blood center for providing the rare subgroup RBCs and Miss Hongwei Zhuang for grammar checking.
References
- Sacks SH, Lennox ES. Monoclonal anti-B as a new blood-typing reagent. Vox Sang. 1981;40:99–104. doi: 10.1111/j.1423-0410.1981.tb00677.x
- Lévy M, Edelman L, Dighiero G. Molecular characterization of a monoclonal murine anti-blood group A antibody. Immunol Lett. 2001;76:15–23. doi: 10.1016/S0165-2478(00)00312-6
- Wittig OL, Alonso JA, Romano EL, Montano RF. A and B blood group-specific monoclonal antibodies. Production and evaluation as ABO-blood typing reagents. Invest Clin. 2006;47:253–64.
- Thorpe SJ, Fox B, Heath AB, Scott M, de Haas M, Kochman S, et al. International standards for minimum potency of anti-A and anti-B blood grouping reagents: evaluation of candidate preparations in an international collaborative study. Vox Sang. 2006;91:336–44. doi: 10.1111/j.1423-0410.2006.00834.x
- Roback JD, Barclay S, Hillyer CD. An automatable format for accurate immunohematology testing by flow cytometry. Transfusion 2003;43:918–27. doi: 10.1046/j.1537-2995.2003.t01-1-00433.x
- Lapierre Y, Rigal D, Adam J, Josef D, Meyer F, Greber S, et al. The gel test: a new way to detect red cell antigen-antibody reactions. Transfusion 1990;30:109–13. doi: 10.1046/j.1537-2995.1990.30290162894.x
- Guo HC, Armstrong VW, Luc G, Billardon C, Goulinet S, Nustede R, et al. Characterization of five mouse monoclonal antibodies to apolipoprotein[a] from human Lp[a]: evidence for weak plasminogen reactivity. J Lipid Res. 1989;30:23–37.
- Abhyankar AV, Gandhi N, Panchal P, Iyer Y, Patel M. Indigenously developed monoclonal antibody specific for human blood group B. J Hematol Malig. 2012;2:18–24.
- Pins MR, Saidman SL, Cosimi AB, Jennings LD, Stowell CP. Accelerated acute rejection of an apparent A2 renal allograft in an O recipient: report of a case with flow cytometric analysis. Transplantation 1997;63:984–8. doi: 10.1097/00007890-199704150-00014
- Hult AK, Olsson ML. Many genetically defined ABO subgroups exhibit characteristic flow cytometric patterns. Transfusion. 2010;50:308–23. doi: 10.1111/j.1537-2995.2009.02398.x
- Takekoshi M, Maeda F, Tachibana H, Inoko H, Kato S, Takakura I, et al. Human monoclonal anti-HCMV neutralizing antibody from phage display libraries. J Virol Methods 1998;74:89–98. doi: 10.1016/S0166-0934(98)00072-X
- Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008
- McGowan A, Tod A, Chirnside A, Green C, McColl K, Moore S, et al. Stability of murine monoclonal anti-A, anti-B and anti-A,B ABO grouping reagents and a multi-centre evaluation of their performance in routine use. Vox Sang. 1989;56:122–30. doi: 10.1111/j.1423-0410.1989.tb04964.x
- Marsh WL. Scoring of hemagglutination reactions. Transfusion 1972;12:352–3. doi: 10.1111/j.1537-2995.1972.tb04459.x
- Oguchi Y, Kawaguchi T, Suzuta T, Osawa T. The nature of human blood group A3 erythrocytes. Vox Sang. 1978;34:32–39. doi: 10.1111/j.1423-0410.1978.tb02877.x
- Yokoyama M, Stacey SM, Dunsford I. Bx – a new sub-group of the blood group B. Vox Sang. 1957;2:348–56. doi: 10.1111/j.1423-0410.1957.tb03957.x
- Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–4. doi: 10.1093/nar/gku316
- Wallace IM, O'Sullivan O, Higgins DG, Notredame C. M-Coffee: combining multiple sequence alignment methods with T-Coffee. Nucleic Acids Res. 2006;34:1692–9. doi: 10.1093/nar/gkl091
- Moretti S, Armougom F, Wallace IM, Higgins DG, Jongeneel CV, Notredame C. The M-Coffee web server: a meta-method for computing multiple sequence alignments by combining alternative alignment methods. Nucleic Acids Res. 2007;35:W645–8. doi: 10.1093/nar/gkm333
- Chen HT, Kabat EA, Lundblad A, Ratcliffe RM. Nucleotide and translated amino acid sequences of cDNA coding for the variable regions of the light and heavy chains of mouse hybridoma antibodies to blood group A and B substances. J Biol Chem. 1987;262:13579–83.
- Nickerson KG, Tao MH, Chen HT, Larrick J, Kabat EA. Human and mouse monoclonal antibodies to blood group A substance, which are nearly identical immunochemically, use radically different primary sequences. J Biol Chem. 1995;270:12457–65. doi: 10.1074/jbc.270.21.12457
- Garratty G, Arndt PA. Applications of flow cytofluorometry to red blood cell immunology. Cytometry 1999;38:259–67. doi: 10.1002/(SICI)1097-0320(19991215)38:6<259::AID-CYTO1>3.0.CO;2-P
- Le Pendu J, Lambert F, Samuelsson B, Breimer ME, Christiane Seitz R, Urdaniz MP, et al. Monoclonal antibodies specific for type 3 and type 4 chain-based blood group determinants: relationship to the A1 and A2 subgroups. Glycoconjugate J. 1986;3:255–71. doi: 10.1007/BF01051776
- Cartron JP, Gerbal A, Hughes-Jones NC, Salmon C. ‘Weak A’ phenotypes. Relationship between red cell agglutinability and antigen site density. Immunology 1974;27:723–7.