ABSTRACT
Objectives: To clarify optimal strategies for human leukocyte antigen (HLA)-mismatched haploidentical hematopoietic stem cell transplantation (HSCT).
Methods: Twelve patients who underwent HSCT from a haploidentical related donor using low-dose thymoglobulin were analyzed retrospectively. Thymoglobulin was added to conditioning regimens at 2.5 mg/kg/day for 2 days (days −4 and −3). Prophylaxis against graft-versus-host disease (GVHD) was performed with cyclosporine and methotrexate.
Results: The median age of the patients was 33 years. Six patients had previous allogeneic HSCT, and HSCT was performed in non-remission for nine patients. All patients but one, who died due to early infection, achieved neutrophil engraftment at a median of 17 days with complete donor-type chimerism. Acute and chronic GVHD were observed in six and five patients, respectively, but no patients died of GVHD-associated complication. No one developed cytomegalovirus disease, but Epstein–Barr virus-related lymphoproliferative disorder was observed in one patient. Long-term survival in remission without immunosuppressive agents are observed in two patients who underwent HSCT in remission, but the majority of patients who underwent HSCT in non-remission experienced disease progression.
Conclusion: Haploidentical HSCT could be performed with thymoglobulin at 5 mg/kg, with the balance between GVHD and relapse rate. The dose reduction of thymoglobulin may be considered for advanced hematological malignancy.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) from a human leukocyte antigen (HLA) 2- or 3-loci-mismatched haploidentical donor is becoming a promising treatment option in patients with hematological malignancy who do not have an HLA-matched sibling [Citation1–5]. The high and prompt availability of a haploidentical donor within a family supports this strategy. Cord blood grafts have a similar feature, but the small amount of nucleated cells in each graft and the inability to acquire donor cells for cell therapy after HSCT might be a disadvantage in adult patients, especially in those with advanced disease.
Various strategies have been used to overcome the high rate of rejection and severe graft-versus-host disease (GVHD) in haploidentical HSCT. Recently, several studies have demonstrated that posttransplantation cyclophosphamide is both safe and effective in haploidentical HSCT [Citation5,Citation6]. However, the relatively high relapse rate for high-risk disease in these studies suggests that this strategy has not sufficiently made use of the potent graft-versus-tumor effect of haploidentical HSCT, and the optimal treatment method for haploidentical HSCT has not been established.
Anti-thymocyte globulin (ATG) is a polyclonal antibody against human thymocyte generated in animals. ATG has been shown to effectively prevent GVHD when it is added to pretransplantation conditioning regimens in allogeneic HSCT from an unrelated donor [Citation7], and recently, even in haploidentical HSCT [Citation2,Citation8–12]. However, increased rates of infection and relapse due to excessive immune suppression might be a problem in this strategy. Therefore, it is very important to identify the optimal usage of ATG (including preparations, doses, and timing) to prevent GVHD, while minimizing the suppression of the immune system [Citation13]. We have used rabbit ATG, thymoglobulin, at a low dose of 5 mg/kg in pretransplantation conditioning regimens since 2009, and in this study we retrospectively evaluated this strategy.
Patients and methods
Patients
The medical records of patients who underwent allogeneic HSCT from a haploidentical related donor using low-dose thymoglobulin (Genzyme, Cambridge, MA, USA) in our institution between October 2009 and April 2015 were evaluated retrospectively. This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University.
Conditioning regimens
The conditioning regimens included myeloablative and reduced-intensity regimens. Patients who underwent a first allogeneic HSCT received a myeloablative regimen that consisted of cyclophosphamide (60 mg/kg for 2 days) and total body irradiation (TBI; 2 Gy twice daily for 3 days). Patients who had a previous history of allogeneic HSCT or were aged more than 55 years received reduced-intensity regimens that consisted of intravenous busulfan (3.2 mg/kg once daily for 4 days) and melphalan (80 mg/m2 for a day), fludarabine (30 mg/m2 for 6 days), busulfan (3.2 mg/kg once daily for 2 days), and TBI (2 Gy twice daily for a day), or fludarabine (25 mg/m2 for 5 days), melphalan (40 mg/m2 for 2 days), and TBI (2 Gy twice daily for a day). Thymoglobulin was added to each regimen at 2.5 mg/kg for 2 days (days −4, and −3). To prevent acute infusion-related reactions to thymoglobulin, patients were pretreated with 1 mg/kg of methyl-prednisolone. Thymoglobulin was infused over 6 hours. On the first day of thymoglobulin infusion, 2.5 mg of thymoglobulin was infused over one hour, and after confirming that no severe infusion-related toxicities were observed, we infused the remaining thymoglobulin over the next 6 hours.
Other transplantation procedures
With regard to the stem cell source, peripheral blood was exclusively used. GVHD prophylaxis consisted of CSA and short-term methotrexate (MTX). CSA was started on day −1 at a dose of 3 mg/kg/day by 24-hour continuous infusion, and the dose was adjusted to maintain the blood concentration between 450 and 550 ng/mL. The dose of MTX was 15 mg/m2 on day 1, and 10 mg/m2 on days 3, 6, and 11. The route of CSA administration was converted to oral at a ratio of 1:2 when patients were able to tolerate oral intake after engraftment. In patients without GVHD, we started tapering CSA between days 30 and 50 by 10% a week and discontinued CSA between days 100 and 120. Acute and chronic GVHD were graded as previously described [Citation14–16].
Granulocyte engraftment was defined as the first day of a neutrophil count of >0.5 × 109 /l for three consecutive days. For platelet engraftment, the platelet count needed to exceed 20 × 109/l without transfusion for 7 days.
Chimerism and immune reconstitution
Chimerism analysis was performed by sex-chromosome fluorescence in situ hybridization (FISH) or the short tandem repeat method using bone marrow cells. Immune reconstitution was evaluated by the quantification of CD3+/CD4+ cells and CD3+/CD8+ cells by flowcytometry. The mean value was calculated using the cell counts at 1 month ± 7 days, 2 and 3 months ± 14 days, 6 months ± 28 days, and 12 months ± 90 days after HSCT.
Statistical considerations
Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan–Meier method, whereas the relapse/progression rate (RR) and non-relapse/progression mortality (NRM) were calculated using Gray’s method considering each other event as a competing risk [Citation17]. These statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University) [Citation18], which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0, Vienna, Austria).
Results
Patients and donors
A total of 12 patients underwent haploidentical HSCT in our institution between October 2009 and April 2015. The patients consisted of five patients with acute myelogenous leukemia, three with acute lymphoblastic leukemia, and one with chronic myelomonocytic leukemia, non-Hodgkin lymphoma, Langerhans cell sarcoma, and primary myelofibrosis, respectively. A patient with primary myelofibrosis was diagnosed with intermediate-1 according to the dynamic international prognostic scoring system [Citation19], and he had transfusion-dependent anemia. Their median age was 33 years (range 15–64 years). Six patients had previous allogeneic HSCT, and two of them received HSCT from the same donor as in the previous HSCT. HSCT was performed in non-remission for nine patients. No patients had donor-specific HLA antibodies. The characteristics of the patients are summarized in .
Table 1. Patient characteristics.
Seven donors were siblings, three were fathers, and two were sons. Their median age was 36 years (range 22–60 years). The median numbers of CD34+ cells and CD3+ cells in the graft were 3.4 × 106/kg (range 3.0–5.7 × 106/kg) and 1.5 × 108/kg (range 0.5–3.2 × 108/kg), respectively. The characteristics of the donors are summarized in .
Table 2. Donor characteristics.
Transplantation outcomes
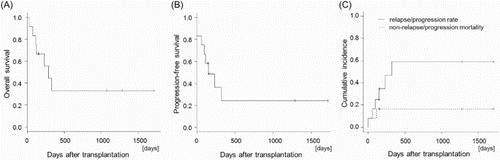
All patients but one, who died due to infection on day 27, achieved neutrophil engraftment at a median of 17 days (range 15–73 days) after HSCT with complete donor-type chimerism. Nine patients achieved platelet counts of 20 × 109/l or higher at a median of 26 days (range 24–80 days) after HSCT. In all patients, OS at 1 year was 33.3% ((a)). PFS, RR, and NRM at 1 year were 24.3%, 59.0%, and 16.7%, respectively ((b) and (c)). Two of the three patients who underwent HSCT in remission are still alive in remission without immunosuppressive agents at days 1280 and 1711, respectively. The other patient who underwent HSCT as a second HSCT relapsed at day 321, but this patient is still alive in remission following a third HSCT at day 1077 after the second HSCT. Seven of the nine patients who underwent HSCT in non-remission achieved complete remission (CR) after HSCT. However, four of these patients relapsed and died due to disease progression. Another patient could not continue cyclosporine with the adverse effect of massive fluid retention, and died due to the idiopathic pneumonia syndrome at day 113 after HSCT.
Figure 1. Transplantation outcomes. Overall survival (A), progression-free survival (B), and the relapse/progression rate and non-relapse/progression mortality (C) from transplantation in all patients.
Acute GVHD was observed in six patients, four with grade I (skin stages 1 or 2), one with grade II (skin stage 3), and the other with grade III (skin stage 2 and gut stage 3). Chronic GVHD was observed in five patients: two with limited disease and three with extensive disease. Extensive disease in two patients, one with mild mouth and skin lesions and one with moderate gastrointestinal tract lesion, improved following the dose adjustments of immunosuppressive agents. In the other patient, extensive chronic GVHD developed after relapse and the rapid tapering of immunosuppressive agents.
Cytomegalovirus (CMV) antigenemia assay became positive in five patients, but none developed CMV disease. In one patient, Epstein–Barr virus (EBV)-related lymphoproliferative disorder (EBV-LPD) was observed on day 52 during the treatment of grade III acute GVHD with steroid. It disappeared with the 6-times use of rituximab. In addition, high EBV-deoxyribonucleic acid (DNA) load (more than 1 × 104 copies/mL) without EBV-LPD was observed in three patients, but all improved with the only one-time use of rituximab. One of them also had hemorrhagic cystitis due to adenovirus, and it improved with the use of cidofovir. These transplantation outcomes are summarized in .
Table 3. Transplantation outcomes.
Immune reconstitution
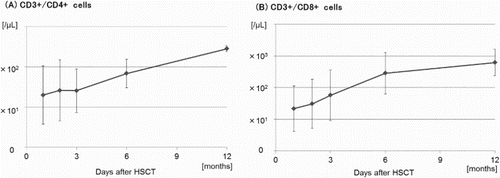
Immune reconstitution was evaluated in nine of the ten patients who achieved CR after HSCT (). Geometric mean numbers of CD3+/CD4+ cells and CD3+/CD8+ cells were 25.4/µL and 57.7/µL at 90 days after HSCT and 68.3/µL and 286.3/µL at 180 days after HSCT, respectively.
Figure 2. Immune reconstitution. Immune reconstitution was evaluated by the quantification of CD3+/CD4+ and CD3+/CD8+ cells by flowcytometry in nine of ten patients who achieved CR after allogeneic hematopoietic stem cell transplantation (HSCT). The geometric mean values were calculated using the cell counts at 1 month, 2 months, 3 months, 6 months, and 12 months after HSCT.
Discussion
Thymoglobulin is a rabbit ATG preparation, and is the only ATG preparation that is covered by Japanese medical insurance for pretransplantation conditioning. Thymoglobulin is believed to induce immunosuppression through in vivo T-cell depletion, and this effect would depend on the doses of thymoglobulin. In haploidentical HSCT, thymoglobulin has been administered at a total dose of 5–12 mg/kg in pretransplantation conditioning [Citation2,Citation9–12]. Previously, we demonstrated that the administration of ATG at 10 mg/kg in patients with aplastic anemia led to persistent severe cell-mediated immune suppression [Citation13], and this dose of thymoglobulin was thought to be too immunosuppressive in HLA-matched HSCT. On the other hand, Kim et al. reported the successful prevention of severe GVHD using thymoglobulin at 2.5 mg/kg in pretransplantation conditioning from an HLA-2 loci mismatched unrelated donor without a significant increase in CMV disease [Citation7]. Therefore, we performed haploidentical HSCT using thymoglobulin at 5 mg/kg (2.5 mg/kg/day for days −4 and −3). In this strategy, some patients developed grades II–IV acute GVHD and extensive chronic GVHD, but none of the patients died of GVHD-associated complications. In addition, the two patients who survived without relapse became free form immunosuppressive agents at 185 days and 173 days after HSCT. Moreover, none of the patients had CMV disease. As described in the study by Cho et al. [Citation11], 5 mg/kg of thymoglobulin is enough to prevent severe GVHD, and is not excessive for inducing CMV disease.
Attention should be paid to the fact that one and three patients had EBV-LPD and high EBV-DNA load, respectively. Close monitoring of EBV-DNA load is required in haploidentical HSCT using low-dose thymoglobulin. The administration of rituximab was effective in all patients.
Nine patients underwent HSCT in non-remission, and five of the seven patients, excluding two patients who died due to infection at 27 days and idiopathic pneumonia syndrome at 113 days after HSCT without relapse or progression, experienced relapse or progression within a year after HSCT. We should consider reducing thymoglobulin to a further lower dose in these patients with advanced hematological malignancy to enhance the graft-versus-tumor effect.
Immune reconstitution after HSCT in our study might be slower than that in the study by Cho et al. [Citation11], where the mean number of CD3+/CD4+ cells at 6 months was greater than 100/µL using thymoglobulin at 2.5 mg/kg. Our results might even be slower than those in the study by Lee et al. [Citation10], which used thymoglobulin at 12 mg/kg. This difference might be attributed to the lower numbers of CD34+ cells and CD3+ cells in the graft in our study. An increase in CD34+ cells and CD3+ cells in the graft and the addition of CD3+ cells by donor lymphocyte infusion might promote early immune reconstitution and a potent graft-versus-tumor effect after HSCT. On the other hand, recovery of CD3+ cells after 3 months might be associated with extensive chronic GVHD in some patients. We should be careful for the dose adjustments of immunosuppressive agents, especially in this period.
In conclusion, haploidentical HSCT could be performed with thymoglobulin at a total dose of 5 mg/kg, with the balance between GVHD and relapse risk. Moreover, the dose reduction of thymoglobulin may be considered in patients with advanced hematological malignancy.
Prospective studies including larger number of patients are warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr. Shinichi Kako is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is an associate professor of hematology.
Dr. Yu Akahoshi is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a clinical associate of hematology.
Dr. Naonori Harada is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a clinical associate of hematology.
Dr. Hirofumi Nakano is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a clinical associate of hematology.
Dr. Kazuaki Kameda is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a research associate of hematology.
Dr. Tomotaka Ugai is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a graduate student of hematology.
Dr. Ryoko Yamasaki is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a clinical associate of hematology.
Dr. Hidenori Wada is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a research associate of hematology.
Dr. Yuko Ishihara is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a graduate student of hematology.
Dr. Koji Kawamura is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a graduate student of hematology.
Dr. Kana Sakamoto is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a postdoctoral researcher of hematology.
Dr. Miki Sato is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a research associate of hematology.
Dr. Masahiro Ashizawa is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a research associate of hematology.
Dr. Kiriko Terasako-Saito is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a research associate of hematology.
Dr. Shun-ichi Kimura is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is an assistant professor of hematology.
Dr. Misato Kikuchi is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a research associate of hematology.
Dr. Hideki Nakasone is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is an assistant professor of hematology.
Dr. Rie Yamazaki is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. She is a research associate of hematology.
Dr. Junya Kanda is a faculty member at Saitama Medical Center, JichiMedical University, Saitama, Japan. He is an assistant professor of hematology.
Dr. Yoshinobu Kanda is a faculty member at Saitama Medical Center, Jichi Medical University, Saitama, Japan. He is a professor of hematology.
Additional information
Funding
References
- Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–3454. doi: 10.1200/JCO.2005.09.117
- Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107(8):3065–3073. doi: 10.1182/blood-2005-05-2146
- Ogawa H, Ikegame K, Yoshihara S, et al. Unmanipulated HLA 2-3 antigen-mismatched (haploidentical) stem cell transplantation using nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12(10):1073–1084. doi: 10.1016/j.bbmt.2006.06.007
- Kanda Y, Oshima K, Kako S, et al. In vivo T-cell depletion with alemtuzumab in allogeneic hematopoietic stem cell transplantation: combined results of two studies on aplastic anemia and HLA-mismatched haploidentical transplantation. Am J Hematol. 2013;88(4):294–300. doi: 10.1002/ajh.23392
- Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005
- Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–122. doi: 10.1016/j.bbmt.2012.08.014
- Kim HJ, Min WS, Cho BS, et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2009;15(6):704–717. doi: 10.1016/j.bbmt.2009.02.010
- Ogawa H, Ikegame K, Kaida K, et al. Unmanipulated HLA 2-3 antigen-mismatched (haploidentical) bone marrow transplantation using only pharmacological GVHD prophylaxis. Exp Hematol. 2008;36(1):1–8. doi: 10.1016/j.exphem.2007.08.013
- Huang XJ, Liu DH, Liu KY, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(2):257–265. doi: 10.1016/j.bbmt.2008.11.025
- Lee KH, Lee JH, Kim DY, et al. Reduced-intensity conditioning therapy with busulfan, fludarabine, and antithymocyte globulin for HLA-haploidentical hematopoietic cell transplantation in acute leukemia and myelodysplastic syndrome. Blood. 2011;118(9):2609–2617. doi: 10.1182/blood-2011-02-339838
- Cho BS, Yoon JH, Shin SH, et al. Comparison of allogeneic stem cell transplantation from familial-mismatched/haploidentical donors and from unrelated donors in adults with high-risk acute myelogenous leukemia. Biol Blood Marrow Transplant. 2012;18(10):1552–1563. doi: 10.1016/j.bbmt.2012.04.008
- Walker I, Shehata N, Cantin G, et al. Canadian multicenter pilot trial of haploidentical donor transplantation. Blood Cells Mol Dis. 2004;33(3):222–226. doi: 10.1016/j.bcmd.2004.08.006
- Terasako K, Sato K, Sato M, et al. The effect of different ATG preparations on immune recovery after allogeneic hematopoietic stem cell transplantation for severe aplastic anemia. Hematology. 2010;15(3):165–169. doi: 10.1179/102453309X12583347113852
- Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001
- Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004
- Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. doi: 10.1182/blood-2009-09-245837
