ABSTRACT
Objective: This study investigated the efficacy and safety of low-dose lenalidomide combined with dexamethasone in elderly patients with relapsed and refractory multiple myeloma (MM).
Methods: Thirty-two elderly patients with refractory and recurrent MM (median age: 64 years) were treated with low-dose lenalidomide (LD-R) combined with dexamethasone (D). LD-R (10 mg/d) was administered orally for 21 days and D (40 mg/d) was administered twice a day on days 1–4, 9–12, and 17–20. The treatment lasted 2–8 28-day cycles.
Results: After two cycles, the complete, very good partial, and partial remission rates were 12.5% (4/32), 25.0% (8/32), and 34.4% (11/32), respectively. The overall response rate was 71.9% (23/32). After a 24-month follow-up, 23 patients responded to therapy, three were in complete remission, four were stable, and 16 exhibited disease progression. In addition, median time-to-progression was 13 months. Observed side effects were hypodynamia, gastrointestinal reaction, peripheral neuritis, and mild hypocytosis.
Conclusion: Low-dose lenalidomide in combination with dexamethasone is an effective and safe treatment for relapsed and refractory MM in elderly patients.
Introduction
Multiple myeloma (MM) is a hematological malignancy of plasma cells and predominantly a disease of the elderly because the incidence of MM increases with age [Citation1,Citation2]. At present, MM cannot be cured completely. Advanced age is an important factor for poor prognosis among patients with MM. Additional factors contributing to poor prognosis in elderly patients are complications, poor performance status at diagnosis, insufficient treatment, decreased physiological reserve, and low expectations of treatment success. Therefore, elderly patients manifest recurrent and refractory MM, the treatment of which presents a substantial challenge [Citation3,Citation4].
New anti-myeloma drugs have improved efficacy and reduced side effects and help in improving the prognosis of patients with MM [Citation5,Citation6]. Immunomodulatory drugs, including thalidomide, lenalidomide, and bortezomib, are effective in treating refractory and recurrent myeloma [Citation7–9]. Thalidomide is widely used in MM; however, it has many adverse reactions [Citation10]. Lenalidomide, a second-generation immunomodulatory drug, is a derivative of thalidomide. Lenalidomide enhances immunomodulatory effects and overcomes the adverse events of thalidomide. Chanan-Khan et al. [Citation11] found that lenalidomide in combination with dexamethasone prolonged the median progression-free survival and median time-to-progression in patients >65 years of age with relapsed or refractory MM. However, routine-dose lenalidomide combined with chemotherapy leads to severe adverse events, including hypodynamia and thromboembolism, especially in elderly patients aged >60 years, with poor tolerability after receiving many cycles of chemotherapy.
In this study, we designed a low-dose lenalidomide in combination with dexamethasone drug regimen to treat elderly patients with refractory and recurrent MM to reduce adverse events, increase tolerability, and further enhance efficacy and prognosis.
Materials and methods
General data
Thirty-two elderly patients with refractory and recurrent MM were enrolled between January 2010 and June 2014. The study included eight refractory patients and 24 recurrent patients (18 male, 14 female), with median age of 64 years (range: 60–76 years). Moreover, nine cases were with IgG-κ, seven cases with IgG-λ, five cases with IgA-κ, four cases with IgA-λ, one case with IgE-κ, four cases with κ light chain, and two cases with λ light chain. According to ISS staging, 7, 3, 19, and 3 cases were in II A, II B, III A, and III B phases, respectively. The median duration was 11 months (range 3–19 months). Median number of previous chemotherapy cycles was 5 (range 2–10), including vincristine, adriamycin, and dexamethasone (VAD); doxorubicin hydrochloride liposomes, vincristine, and dexamethasone (DVD); bortezomib and dexamethasone (BD); thalidomide and dexamethasone (TD); melphalan and prednisone (MP); melphalan, prednisone, and thalidomide (MPT); cyclophosphamide, vincristine, lomustine, melphalan, and prednisone (M2); and dexamethasone, thalidomide, cisplatin, adriamycin, cyclophosphamide, and etoposide (DT-PACE). Patient characteristics are summarized in . Patients with comorbidities such as hypertension, diabetes, and gastric ulcers were excluded.
Table 1. Baseline patient demographics and disease characteristics (n = 32).
Treatment protocol
Chemotherapy regimen contained a low dose of lenalidomide (LD-R) in combination with dexamethasone (D). Patients orally received LD-R (10 mg/d), on days 1–21 of each 28-day cycle, in combination with D (40 mg/d), on days 1–4, 9–12, and 17–20. Patients underwent 2–8 28-day cycles. In hyperglycemic patients on insulin, the dose of dexamethasone was reduced to 20 mg/d. After two cycles, bone marrow cytomorphological examination, serum protein electrophoresis, immunofixation electrophoresis, serum-free light chain test, 24-hour urine volume test, biochemistry analysis, blood and urine parameters, electrocardiogram, and X-ray were performed. If patients responded to treatment, the therapy was continued for 6–8 cycles, otherwise, the treatment was aborted. The patients were examined every two months, at the point of stopping the treatment, or after 6–8 cycles, with a follow-up for 2 years.
Assessment of efficacy and adverse events
Efficacy was estimated as previously described [Citation12]. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for adverse events, version 3.0.
Results
Clinical efficacy and follow-up
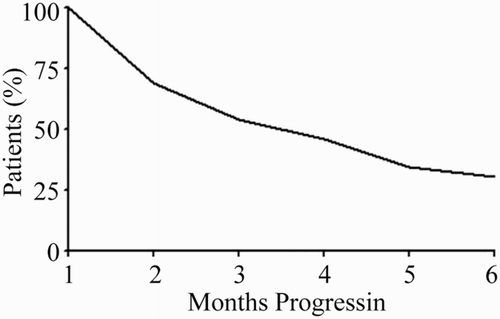
shows treatment cycles for the 32 patients. After two cycles, the complete, very good partial, and partial remission rates were 12.5% (4/32), 25.0% (8/32), and 34.4% (11/32), respectively, with the overall response rate (ORR) of 71.9% (23/32). After 24-month follow-up, 23 patients presented a response: three patients were in complete remission, four were stable, and 16 patients exhibited disease progression. The median time-to-progression was 13 months; nine patients did not show any response to treatment; however, after changing treatment regimens, two patients were stable and seven showed disease progression. shows time to progression.
Table 2. Courses of LD-RD regime in patients with MM (n = 32).
Adverse reactions
Adverse reactions observed included fatigue, pyrexia, peripheral neuropathy, neutropenia, and hyperglycemia. However, the adverse reactions were mild and no grade 4 adverse reactions occurred (). Two cases presented neutropenia, which caused severe lung infection. The infection was controlled after administration of anti-infectious drugs and granulocyte colony-stimulating factor. Because of use of multiple therapy cycles and large hormone doses, hyperglycemia occurred in 15 patients, five of whom required insulin administration.
Table 3. Adverse effects (n = 32).
Discussion
Although the remission rate and survival of patients with MM can be improved to some degree [Citation13], myeloma cells easily develop drug resistance, causing refractory and recurrent MM, for which no curative treatment has been presently established, especially in elderly patients. Many patients display poor tolerability to stem cell transplantations, which leads to low ORR and poor prognosis. New immunomodulatory drugs, including thalidomide, lenalidomide, and bortezomib (a proteasome inhibitor), have significantly improved the clinical prognosis of patients with MM when compared with traditional therapy [Citation14].
Lenalidomide is a thalidomide analog that exerts pleiotropic effects, including antiangiogenic and antineoplastic activity and induction of cell apoptosis. Compared to thalidomide, lenalidomide has more stable chemical properties and stronger anti-tumor and immunomodulatory activity and overcomes the common adverse events of thalidomide. Richardson et al. [Citation14] used 25 mg/d lenalidomide single therapy to treat 27 recurrent and refractory MM patients, with the ORR of 71% in 24 patients with MM, and reports of grade 3 and 4 granulocytopenia and thrombocytopenia. A phase I/II clinical trial using lenalidomide to treat recurrent and refractory MM showed that 29% patients receiving lenalidomide were without remission; however, patients who received lenalidomide in combination with dexamethasone reached remission. Thus, lenalidomide combined with other drugs is explored as a new therapy regimen to treat MM by many researchers. Weber et al. [Citation15] performed a multicenter, double-blind, placebo-controlled, randomized, phase III trial that compared lenalidomide plus dexamethasone with placebo plus dexamethasone in 353 patients with relapsed or refractory MM. The results showed that lenalidomide plus dexamethasone was superior to high-dose dexamethasone alone in terms of the ORR (60.6% vs. 21.9%). In addition, the complete remission rate in the experimental group was higher than that in the placebo group (15% vs. 2%), and the overall survival was significantly longer for patients in the experimental group (38 months) than for those in the placebo group (31.6 months). Furthermore, Joao et al. [Citation16] used lenalidomide plus dexamethasone for the treatment of relapsed and refractory MM by evaluating 90 consecutive patients treated in their center. The ORR to this treatment was 68%; however, some side effects were observed. In these regimens, all patients received 25 mg/d lenalidomide on days 1–21. Additionally, Weber et al. indicated that grade 3 or 4 hematologic toxic effects occurred in 52.5% of patients in the lenalidomide group, and that grade 3 or 4 incidence of infection was 21.5% in the lenalidomide group. In addition, grade 3 or 4 peripheral neuropathy, diarrhea, and constipation occurred. Incidence of grade 3 or 4 thromboembolic events was 14.7%, and 35 out of 177 (19.8%) patients who received lenalidomide stopped the trial because of adverse events. Joao et al. found similar side effects [Citation15,Citation16].
Owing to racial differences, the dose of lenalidomide is sometimes reduced to 10–15 mg/d [Citation17,Citation18]. In this study, elderly patients were enrolled (median age: 64 years). Many patients received more than two drug regimens for the treatment of MM and had poor prognosis. Therefore, low-dose lenalidomide plus dexamethasone regimen (LD-RD) was applied. Results show that all patients tolerated the treatment well, with the median of six cycles. Importantly, thromboembolic events and grade 4 adverse events did not occur. Grade 3 adverse events occurred only in a few patients, including fatigue (3.13%), pyrexia (6.25%), peripheral neuropathy (3.13%), neutropenia (6.25%), and hyperglycemia (15.63%). However, these adverse events were mild and patients recovered without any treatment or with symptomatic supportive treatment. Our results show that the ORR was 71.89%. The median time to progression was 13 months, similar to the results of Weber et al. Taken together, these results indicate that low-dose lenalidomide plus dexamethasone is an effective and safe therapy for elderly patients with relapsed and refractory MM.
However, the median time to progression was approximately 1 year for low- and routine-dose regimens, and MM was not cured. The development of new drugs, including proteasome inhibitors, histone deacetylase inhibitors, AKT-targeting drugs, and immunomodulatory drugs could improve the prognosis of relapsed and refractory MM further [Citation19,Citation20].
Acknowledgements
This study was conducted in accordance with the declaration of Helsinki and was conducted with approval from the Ethics Committee of Huai’an First People's Hospital, Nanjing Medical University. Written informed consent was obtained from all participants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. [ cited 2012 Jan];87(1):78–88. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Am+J+Hematol+2012%3B+87%3A+78-88 doi: 10.1002/ajh.22237
- Bergsagel PL, Mateos MV, Gutierrez NC, et al. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. [ cited 2013 Feb 7];121(6):884–892. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Improving+overall+survival+and+overcoming+adverse+prognosis+in+the+treatment+of+cytogenetically+high-risk+multiple+myeloma doi: 10.1182/blood-2012-05-432203
- Pulte D, Redaniel MT, Lowry L, et al. Age disparities in survival from lymphoma and myeloma: a comparison between US and England. Br J Haematol. [ cited 2014 Jun];165(6):824–831. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Age+disparities+in+survival+from+lymphoma+and+myeloma%3A+a+comparison+between+US+and+England doi: 10.1111/bjh.12837
- Wildes TM, Rosko A, Tuchman SA. Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. [ cited 2014 Aug 20];32(24):2531–2540. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Multiple+Myeloma+in+the+Older+Adult%3A+Better+Prospects%2C+More+Challenges doi: 10.1200/JCO.2014.55.1028
- Uttervall K, Duru AD, Lund J, et al. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One. [ cited 2014 Jul 8];9(7):e101819. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=The+use+of+novel+drugs+can+effectively+improve+response%2C+delay+relapse+and+enhance+overall+survival+in+multiple+myeloma+patients+with+renal+impairment doi: 10.1371/journal.pone.0101819
- Laubach JP, Voorhees PM, Hassoun H, et al. Current strategies for treatment of relapsed/refractory multiple myeloma. Expert Rev Hematol. [ cited 2014 Feb];7(1):97–111. Available at http://www.ncbi.nlm.nih.gov/pubmed/24471924 doi: 10.1586/17474086.2014.882764
- Kropff M, Baylon HG, Hillengass J, et al. Thalidomide versus dexamethasone for the treatment of relapsed and/or refractory multiple myeloma: results from OPTIMUM, a randomized trial. Haematologica. [ cited 2012 May];97(5):784–791. Available at http://www.ncbi.nlm.nih.gov/pubmed/22133776 doi: 10.3324/haematol.2011.044271
- Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. [ cited 2014 Mar 6];123(10):1461–1469. Available at http://www.ncbi.nlm.nih.gov/pubmed/24429336 doi: 10.1182/blood-2013-07-517276
- Zago M, Oehrlein K, Rendl C, et al. Lenalidomide in relapsed and refractory multiple myeloma disease: feasibility and benefits of long-term treatment. Ann Hematol. [ cited 2014 Dec];93(12):1993–1999. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Lenalidomide+in+relapsed+and+refractory+multiple+myeloma+disease%3A+feasibility+and+benefits+of+long-term+treatment doi: 10.1007/s00277-014-2149-2
- Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. [ cited 2014 Mar 20];123(12):1826–1832. Available at http://www.ncbi.nlm.nih.gov/pubmed/24421329 doi: 10.1182/blood-2013-11-538835
- Chanan-Khan AA, Lonial S, Weber D, et al. Lenalidomide in combination with dexamethasone improves survival and time-to-progression in patients ≥65 years old with relapsed or refractory multiple myeloma. Int J Hematol. [ cited 2012 Aug];96(2):254–262. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Int+J+Hematol+2012%3B+96%3A+254-262 doi: 10.1007/s12185-012-1125-7
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. [ cited 2006 Sep];20(9):1467–1473. Available at http://www.ncbi.nlm.nih.gov/pubmed/16855634 doi: 10.1038/sj.leu.2404284
- Romano A, Conticello C, Cavalli M, et al. Salvage therapy of multiple myeloma: the new generation drugs. Biomed Res Int. [ cited 2014];2014:456037. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Salvage+therapy+of+multiple+myeloma%3A+the+new+generation+drugs.+Biomed+Res+Int+2014%3B+2014%3A+456037
- Richardson P, Mitsiades C, Laubach J, et al. Lenalidomide in multiple myeloma: an evidence-based review of its role in therapy. Core Evid. [ cited 2010 Jun 15];4:215–245. Available at http://www.ncbi.nlm.nih.gov/pubmed/20694078
- Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. [ cited 2007 Nov 22];357(21):2133–2142. Available at http://www.ncbi.nlm.nih.gov/pubmed/18032763 doi: 10.1056/NEJMoa070596
- João C, Coelho I, Costa C, et al. Efficacy and safety of lenalidomide in relapse/refractory multiple myeloma – real life experience of a tertiary cancer center. Ann Hematol. [ cited 2015 Jan];94(1):97–105. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Efficacy+and+safety+of+lenalidomide+in+relapse%2Frefractory+multiple+myeloma-Real+life+experience+of+a+tertiary+cancer+center doi: 10.1007/s00277-014-2164-3
- Shen M, Wang J, Yang G, et al. Effectiveness of lenalidomide-based chemotherapy on 25 patients with multiple myeloma. J Leukemia Lymphoma. [ cited 2010];19(7):401–403. Available at http://d.wanfangdata.com.cn/Periodical/bxblbl201007006
- Zhong Y. Progress in the treatment of lenalidomide on relapsed and refractory multiple myeloma. J Mod Med Health. [ cited 2013];29(10):1514–1517. Available at http://www.xdyyws.com/paper.aspx?id=10966
- Spencer A, Yoon SS, Harrison SJ, et al. Novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. [ cited 2014 Oct 2];124(14):2190–2195. Available at http://www.ncbi.nlm.nih.gov/pubmed/?term=Novel+AKT+inhibitor+afuresertib+shows+favorable+safety%2C+pharmacokinetics%2C+and+clinical+activity+in+multiple+myeloma doi: 10.1182/blood-2014-03-559963
- Castelli R, Orofino N, Losurdo A, et al. Choosing treatment options for patients with relapsed/refractory multiple myeloma. Expert Rev Anticancer Ther. [ cited 2014 Feb];14(2):199–215. Available at http://www.ncbi.nlm.nih.gov/pubmed/24329153 doi: 10.1586/14737140.2014.863153