ABSTRACT
Objective: To gain further insights into the predisposing risk factors for central nervous system (CNS) involvement in patients with acute lymphocytic leukemia (ALL), the impact of CD56 expression in these patients was investigated.
Methods: We reviewed the clinical features of CD56 expression in 588 consecutive ALL patients treated with systemic chemotherapy regimens between 2000 and 2014. The categorical data from CD56+ ALL patients were compared with those from CD56− ALL patients.
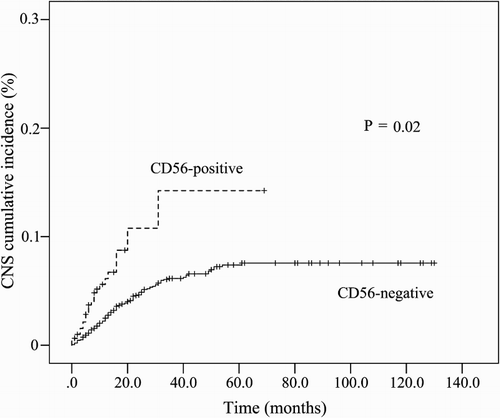
Results: Among the 588 patients studied, 18.9% showed CD56 expression. The expression was significantly associated with CD33+, CD10−, CD15+, TdT−, and CD5+ immunophenotypes. After systemic chemotherapy, 8.8% patients showed CNS involvement, of which 3.2% exhibited combined recurrences and 5.6% exhibited isolated CNS involvement. The 5-year event-free survival was significantly lower for patients with CD56+ immunophenotype compared with patients with CD56− immunophenotype (22.5% vs. 32.7%, P = 0.04). Cumulative incidences of CNS involvement were significantly greater in the CD56+ cohort compared with the CD56− cohort (14.4% vs. 7.5%, P = 0.02). Multivariate analysis revealed CD56 expression to be statistically significant risk factors for CNS involvement.
Conclusion: CD56 expression should be regarded as an independent risk factor for ALL with CNS involvement in adults.
Introduction
Risk-adapted effective systemic chemotherapy with intrathecal chemotherapy has increased the overall survival (OS) for acute lymphocytic leukemia (ALL). However, the involvement of ALL in the central nervous system (CNS) in adults is considered to be a poor outcome [Citation1]. In clinical practice, the management of adults with ALL and CNS involvement has not been standardized, with different perspectives and study results further complicating matters. Risk factors that can predict an inferior outcome in ALL with CNS involvement have been widely investigated, including high initial WBC (white blood cell) count, cytogenetic abnormalities, older age, special ALL subtypes (such as Burkitt’s leukemia), and CNS hemorrhage during induction therapy [Citation2]; however, little progress has been made in this field regarding differences in the cell-surface markers.
Previous studies suggest that leukemic cell expression of CD56, a neural cell-adhesion molecule, is associated with higher incidence of relapse and poorer outcome in both acute myeloid leukemia with t(8;21) and acute promyelocytic leukemia, and may predispose to a higher frequency of CNS involvement [Citation3]. However, the clinical significance of CD56 antigen in ALL has not been well elucidated.
To gain further insights into the risk impact of CD56 expression in ALL with CNS involvement, we analyzed the clinical characteristics and outcomes of a series of adult ALL patients who did not receive cranial irradiation as CNS prophylaxis in Zhejiang and determined the risk factors related to ALL with CNS involvement.
Materials and methods
Patients
From February 2000 to October 2014, we conducted a retrospective study comprising 588 adult patients with newly diagnosed ALL from our hospitals. Eligibility criteria were: (i) The diagnostics were comprised of cytomorphology, cytochemistry, molecular genetics, and immunophenotyping of bone marrow; (ii) older than 18 years old; (iii) patient cooperation with clinical treatment; (iv) patients with CNS involvement at the time of diagnosis were not included in the study. The clinical characteristics of the study cohort are as presented in . The study did not harm patients’ health or privacy. Informed consent was obtained from all participants. The study was approved by the Research Ethics Boards of the first affiliated hospital of Wenzhou medical university and was performed in compliance with the Declaration of Helsinki.
Table 1. Clinical and biological characteristics of ALL according to CD56 expression.
Definitions
The diagnosis and classification of ALL were defined based on the cytomorphology, cytogenetics, molecular biology, and immunophenotyping of bone marrow and were based on the FAB criteria. ALL subtype diagnosis and classification were performed according to the WHO-2008 diagnostic and European Group for the Immunological Characterization of Leukemias (EGIL) criteria [Citation4,Citation5]. This analysis excludes patients with WHO-defined mixed phenotype AL and thus only includes true ALL. Some of ALL patients, however, also expressed one or two myeloid derived antigens – such as CD13, CD15, CD33, and CD117.
Risk stratification was defined according to the Chinese guidelines for the diagnosis and treatment of ALL [Citation6]. None of the patients received cranial radiotherapy as CNS prophylaxis.
CNS involvement was diagnosed based on the presence of leukemic blasts in the centrifuged cerebrospinal fluid (CSF) of patients who had been in complete remission (CR). All patients with evidence of CNS involvement underwent BM (bone marrow) examination at the time of diagnosis. Standard definitions of CR, OS, and event-free survival (EFS) were adopted [Citation7].
With regard to relapse, two groups were defined: (1) isolated CNS relapse (defined based on the presence of blast cells in CSF and not in BM) and (2) CNS and BM relapse (defined by a combined CNS and BM relapse). The median follow-up period was 8.5 years (0–14 years).
Therapy of ALL
It was recommended for all patients to undergo a lumbar puncture examination after the first CR, unless they had signs of CNS involvement or abnormal findings on magnetic resonance imaging requiring an earlier examination.
CNS prophylaxis comprised intrathecal methotrexate (10 mg), cytarabine (50 mg), and dexamethasone (5 mg) with systemic chemotherapy. In patients with BM relapse, a CSF examination was performed to determine CNS involvement.
There are many new chemotherapeutic drugs and treatment protocols being developed. There were no guidelines in routine clinical practice recommending chemotherapy regimens for ALL. In the remission–induction treatment phase, patients at high-risk received four or more drugs [Citation2]. A tyrosine kinase inhibitor was added to multiagent chemotherapy in patients with BCR–ABL1+ ALL [Citation2,Citation8]. Allogeneic hematopoietic stem-cell transplantation is considered for patients with high-risk ALL or persistent disease [Citation9]. Systemic chemotherapy based on the risk group and protocol has been published elsewhere and is summarized in Table S1 [Citation10].
Multiparameter flow cytometry
Immunophenotyping was performed on bone marrow samples collected at ALL diagnosis and analyzed at a reference laboratory using standard immunofluorescence methods. Monoclonal antibodies to the T-lymphoid associated antigens CD2, CD3, CD4, CD5, CD7, and CD8; B-lymphoid-associated antigens CD19, CD10, CD20, CD22, and CD79a; IgM heavy and light chains of immunoglobulins; myeloid-associated antigens antimyeloperoxidase, CD13, CD14, CD11b, CD117, CD15, and CD33; and lineage-nonspecific surface antigens HLA-DR, CD45, TdT, CD34, and CD56 were used to characterize the immunophenotype of leukemic cells. Following the EGIL criteria, surface markers were considered positive if ≥20% of ALL cells expressed a specific antigen [Citation11].
Statistical analysis
Data collected from ALL patients with and without CD56 expression focused on pretreatment characteristics such as clinical characteristics, laboratory data, and cytogenetics and other molecular findings. The categorical data for patients with CD56+ ALL were compared with those of patients with CD56− ALL using chi-square or Fisher’s exact tests. Comparison of the medians of the continuous variables between the two groups was performed using the nonparametric Mann–Whitney U test. Univariate and multivariate Cox logistic regression analyses were used to determine factors predictive of CNS involvement. Variables that met a significance level of <0.2 in univariate analysis were included in multivariate logistic regression analysis. The rate of CNS relapse was estimated using the cumulative incidence method [Citation12]. Cumulative incidences of CNS relapse were measured from the date of CR to the first relapse. OS and EFS of different groups were estimated using the Kaplan–Meier survival analysis and compared by the log-rank test using censored data. Two-sided P values <0.05 were considered statistically different. Analyses were performed using SPSS 12.0 software (SPSS Inc., Chicago, IL).
Results
Patient characteristics according to CD56 expression
This clinical retrospective study included 588 consecutive ALL patients with available data on the analysis of CD56 surface antigen expression at diagnosis between 2000 and 2014. The median age was 36 years (range, 18–76 years). Of the patients, 281 (47.8%) were male and 307 (52.2%) were female. Furthermore, 111 patients (18.9%) showed CD56 expression on leukemic cells. The main clinical characteristics of CD56+ ALL patients are as shown in .
With regard to immunologic phenotype, 66 patients (11.2%) showed an early pre-B phenotype, 306 patients (52.0%) showed a common phenotype, 84 patients (14.3%) showed a pre-B phenotype, 48 patients (8.2%) showed a mature B phenotype, and 84 patients (14.3%) showed a T phenotype. CD56 expression was significantly associated with the CD33+ (P = 0.03), TdT− (P < 0.01), CD5+ (P < 0.01), CD10− (P < 0.01), and CD15+ (P = 0.03) immunophenotypes. There was also a trend toward the CD13+ (P = 0.07) and HLA-DR+ (P = 0.06) immunophenotypes. The immunophenotypic features are as presented in .
Table 2. Immunophenotypic features of ALL patients (n = 588) according to CD56 expression.
There were no statistically significant differences found regarding age, sex, WBC and platelet counts, immunophenotype, bone marrow transplantation, cytogenetic abnormality (BCR–ABL or MLL/AF4), septicemia incidence, and albumin, hemoglobin, fibrinogen, serum LDH, and β2-microglobulin levels. No statistically significant differences were found regarding ALL risk stratification in the comparison between the CD56+ and CD56− populations.
Treatment outcome
The median OS for the entire cohort was 15 months (1–134 months), the median survival of patients with CD56+ ALL cohort and CD56− ALL cohort were 12 and 16 months, respectively. As shown in , 488 of the 588 patients studied achieved hematologic CR (83.0%). The CR rate and early death incidence during treatment were not different between CD56+ and CD56− ALL. After systemic chemotherapy, 52 (8.8%) patients showed CNS involvement, including 19 (3.2%) with combined (CNS and BM) recurrences and 33 (5.6%) with isolated CNS involvement. The EFS was significantly lower for patients with CD56+ ALL compared to those with CD56− ALL (5-year EFS 22.5% vs. 32.7%, P = 0.04).
Table 3. Treatment results according to CD56 expression.
Risk factors associated with CNS involvement
WBC count, > 30 × 109/L (P < 0.01); LDH levels, > 800 U/L (P = 0.03), CD34+ ALL (P = 0.03), and CD56+ ALL (P = 0.02) were variables associated with a greater risk of CNS involvement in univariate analysis. On multivariate analysis, CD56+ ALL retained the independent predictive value along with the initial WBC counts ().
Table 4. Statistically significant variables in univariate and multivariate analysis for CNS relapse.
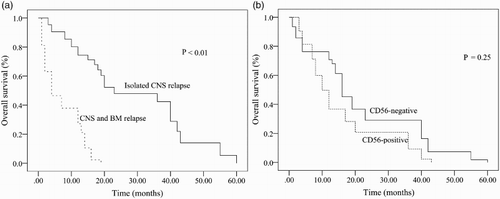
The median survival of patients with CNS involvement was 8 months. Only 11.1% of patients were alive more than 2 years after systemic chemotherapy. The cumulative incidence of CNS relapse was significantly greater in the CD56+ ALL cohort compared with the CD56− ALL cohort (14.4% vs. 7.5%, P = 0.02) (; ). Significant differences in the 5-year OS were observed when patients who exhibited isolated CNS recurrence were compared with patients who exhibited combined recurrence in CNS and BM (P < 0.01) ((a)). However, no differences were observed in the 5-year OS between CD56+ and CD56− patients with CNS involvement ((b)).
Discussion
CNS leukemia continues to be a significant therapeutic challenge in adult ALL patients and has not been solved effectively despite many clinical trials. The routine use of CNS prophylaxis remains the best approach for treating ALL patients with CNS involvement [Citation13]. The inclusion of systematic CNS prophylaxis in adult ALL patients has led to a considerable reduction in CNS involvement to 5–10% [Citation14]. Adult ALL patients with CNS involvement have poor prognosis despite effective intrathecal chemotherapy. The leukemic cell immunophenotype and treatment outcomes in adult ALL patients with CNS involvement have not been well characterized. In the current study, 8.8% of ALL patients showed CNS involvement; these patients showed higher WBC counts (>30 × 109/L) and a more likely association with leukemic cell CD56-positivity than those without CNS involvement (P = 0.01 and P = 0.02, respectively; ). Multivariate analysis showed that CD56 expression had an independent predictive value of CNS involvement.
CD56+ ALL cells frequently coexpressed CD33, CD5, and CD15. Although there are data available on the expression frequency of these surface antigens on ALL blast cells, these antigens have not been implicated as predisposing risk factors for CNS leukemia, with certain studies showing that the aberrant expression of myeloid-associated antigens in ALL cells is associated with poor outcomes [Citation15]. Furthermore, our study shows a significant negative correlation between CD56+ ALL and B-lymphoid associated markers such as CD10 antigen as well as lineage-nonspecific surface antigens such as TdT. The presence of a CD10− ALL immunophenotype was indicative of a poor outcome in a previous study [Citation16]. To the best of our knowledge, no studies have investigated a correlation between TdT− ALL and CNS recurrence in adults. A TdT− ALL immunophenotype may present in Burkitt’s leukemia, which is associated with poor prognosis and CNS involvement [Citation17].
In addition, unlike Paietta et al. [Citation18] in our study, 18.9% patients showed CD56 expression on leukemic cells. Such discrepancy (3.1% vs. 18.9%) might be explained by taking into account the difference criteria for the positivity of cell-surface markers. In Paietta et al. analysis, positivity was defined as specific fluorescence greater than 98% of the isotype control. While, in our analysis, following the EGIL criteria, a sample was defined as positive if ≥ 20% of leukemic cells expressed a specific antigen in the cell surface.
CD56 is also known as neural cell-adhesion molecule (NCAM), which has been associated with shorter remission duration and OS as well as higher cumulative incidences of relapse [Citation19].
The direct molecular mechanism underlying CD56-positivity in ALL leading to a greater risk of relapse still remains unclear. Cell-adhesion molecules represent homing receptors, which play an important role in the targeting of tumors to particular types of tissue (e.g. brain tissue) and development of metastasis [Citation20]. We speculate that CD56+ blast cells have a tendency for intracranial infiltration.
Another possibility has been suggested by a previous report, where adherence and migratory functions in myelocytic-derived leukemic cells are associated with a greater probability of extramedullary leukemia (EML). The authors speculated that the mechanism of EML underlying monocytic-derived AML (FAB subtype M4 or M5) may have involved leukemic cell maturation, allowing early deviation from the BM with subsequent extramedullary tissue involvement [Citation21,Citation22].
In our study, eight patients had initially presented with CD56− ALL (data were provided in Figure S1). These patients achieved CR after systemic chemotherapy and had short DFS but later relapsed with CNS involvement with majority of leukemic cells expressing CD56. This provides further evidence that CD56 expression on leukemic cells may determine a tendency of CNS involvement in ALL.
A high initial WBC count has been considered as an important risk factor for CNS involvement [Citation23]; this was also observed in our study. Higher WBC counts and serum LDH levels correlated with larger leukemic cell burdens, which have been reported to be associated with CNS involvement [Citation23]. However, there were no statistically significant differences between serum LDH levels and CNS involvement after multivariate analysis in our study. Unlike previous reports [Citation24], we were unable to find a significant correlation between CD56 expression and higher WBC counts.
ALL adults who developed CNS involvement in our series had a poor outcome, as described in . In our study, no patient remained alive beyond 5 years after systemic chemotherapy. In patients with CNS involvement, OS for CNS and BM relapse cohort was significantly inferior to that for the isolated CNS relapse cohort, although no differences were observed in OS between CD56+ and CD56− ALL. These findings support previous reports showing that CNS relapse (either isolated or concomitant with BM relapse) is associated with poor outcomes [Citation25].
In conclusion, the presented data suggest that CD56 expression may play an important role in the development of relapse in ALL patients with CNS involvement who were treated with modern systemic chemotherapy. CD56 expression should be regarded as an independent risk factor for ALL with CNS involvement in adults.
S1_Table.zip
Download Zip (1.9 MB)Acknowledgments
We gratefully acknowledge the patient for their participation in our study. We thank Dr Xiao Bo Nie for the critical reading of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes to contributors
Wangqiang Hu, resident doctor; research area: the prognosis of leukemia.
Xiaoxia Wang, associate professor; research area: bone marrow cell morphology.
Rongrong Yang, supervising technician; research area: bone marrow cell morphology.
Laixi Bi, attending doctor; research area: hematological malignancy.
Yaosheng Xie, associate senior technician; research area: bleeding and coagulation.
Zhuo Zhang, supervising technician; research area: platelet function.
Hong Lu, supervising technician; research area: immunology of chronic myeloid leukemia.
Lianfeng Wu, supervising technician; research area: platelet adhesion.
References
- Surapaneni UR, Cortes JE, Thomas D, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia. Cancer. 2002;94(3):773–779. doi: 10.1002/cncr.10265
- Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. doi: 10.1016/S0140-6736(12)62187-4
- Montesinos P, Diaz-Mediavilla J, Deben G, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica. 2009;94(9):1242–1249. doi: 10.3324/haematol.2009.007872
- Matutes E, Pickl WF, Van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011;117(11):3163–3171. doi: 10.1182/blood-2010-10-314682
- Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995;9(10):1783–1786.
- Mi YC. [Expert consensus on adult acute lymphoblastic leukemia in China-interpretation of diagnosis and prognosis stratification]. Zhonghua Xue Ye Xue Za Zhi. 2013;34(11):994–996.
- DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3):526–534. doi: 10.1038/leu.2014.229
- Zhang FH, Ling YW, Zhai X, et al. The effect of imatinib therapy on the outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology. 2013;18(3):151–157. doi: 10.1179/1607845412Y.0000000052
- Marks DI, Alonso L, Radia R. Allogeneic hematopoietic cell transplantation in adult patients with acute lymphoblastic leukemia. Hematol Oncol Clin N. 2014;28(6):995–1009. doi: 10.1016/j.hoc.2014.08.008
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. doi: 10.1200/JCO.2010.30.1382
- Matutes E, Morilla R, Farahat N, et al. Definition of acute biphenotypic leukemia. Haematologica. 1997;82(1):64–66.
- Tai BC, Chen ZJ, Machin D. Estimating sample size in the presence of competing risks – cause-specific hazard or cumulative incidence approach? Stat Methods Med Res. 2015. DOI:10.1177/0962280215623107
- Apostolidou E, Swords R, Alvarado Y, et al. Treatment of acute lymphoblastic leukaemia: a new era. Drugs. 2007;67(15):2153–2171. doi: 10.2165/00003495-200767150-00004
- Ritchey AK, Pollock BH, Lauer SJ, et al. Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: a pediatric oncology group study. J Clin Oncol. 1999;17(12):3745–3752.
- Suggs JL, Cruse JM, Lewis RE. Aberrant myeloid marker expression in precursor B-cell and T-cell leukemias. Exp Mol Pathol. 2007;83(3):471–473. doi: 10.1016/j.yexmp.2007.08.012
- Gleissner B, Goekbuget N, Rieder H, et al. CD10- pre-B acute lymphoblastic leukemia (ALL) is a distinct high-risk subgroup of adult ALL associated with a high frequency of MLL aberrations: results of the German Multicenter Trials for Adult ALL (GMALL). Blood. 2005;106(13):4054–4056. doi: 10.1182/blood-2005-05-1866
- Saikia TK, Gopal R, Nair CN, et al. Central nervous system and testicular involvement in acute lymphoblastic leukaemia. J Assoc Phys India. 1985;33(7):457–459.
- Paietta E, Neuberg D, Richards S, et al. Eastern Cooperative Oncology G. Rare adult acute lymphocytic leukemia with CD56 expression in the ECOG experience shows unexpected phenotypic and genotypic heterogeneity. Am J Hematol. 2001;66(3):189–196. doi: 10.1002/1096-8652(200103)66:3<189::AID-AJH1043>3.0.CO;2-A
- Ito S, Ishida Y, Oyake T, et al. Clinical and biological significance of CD56 antigen expression in acute promyelocytic leukemia. Leukemia Lymphoma. 2004;45(9):1783–1789. doi: 10.1080/10428190410001683624
- Sher BT, Bargatze R, Holzmann B, et al. Homing receptors and metastasis. Adv Cancer Res. 1988;51:361–390. doi: 10.1016/S0065-230X(08)60226-2
- Saiki I, Fujii H, Yoneda J, et al. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54(1):137–143. doi: 10.1002/ijc.2910540122
- van der Weide M, Imandt LM, Langenhuijsen MM. Motility of leukaemic cells in acute leukaemia related to tumour mass, maturation and FAB classification. Acta Haematol. 1988;80(1):34–39. doi: 10.1159/000205595
- Karimi M, Yarmohammadi H, Sabri MR. An analysis of prognostic factors and the five-year survival rate in childhood acute lymphoblastic leukemia. Med Sci Monitor. 2002;8(12):CR792–6.
- Ferrara F, Morabito F, Martino B, et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2000;18(6):1295–1300.
- Reman O, Pigneux A, Huguet F, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis and/or at first relapse: results from the GET-LALA group. Leukemia Res. 2008;32(11):1741–1750. doi: 10.1016/j.leukres.2008.04.011