ABSTRACT
Objectives: Adult patients with refractory/relapsed ALL have poor survival outcomes with current chemotherapies. We aimed to determine safety and efficacy of lenalidomide, an oral immunomodulator, in these patients.
Methods: This phase 1/2 trial (EUDRACT # 2009-009372-13) included 10 patients who received 28-day cycles of oral lenalidomide 25 mg/day, days 1 through 21, in combination with oral dexamethasone 40 mg/day on days 1, 8, 15, 22. Primary endpoints were tolerance and the overall response rate (ORR). Secondary endpoints included overall survival (OS) and quality of life.
Results: The most common grade 3 or 4 adverse events were myelosuppression. The ORR among the participants who could be evaluated was 28.6% (95% confidence interval [CI], 0–62.2%). The median OS was 92 days (range, 43–133 days). All patients have died because of progressive disease. Quality of life remains stable during treatment cycles.
Discussion and conclusion: The safety of combination therapy consisting of lenalidomide plus dexamethasone is consistent with ambulatory administration. Efficacy should be reevaluated in a larger series including patients less intensively previously treated.
Introduction
Most therapeutic advances in adult acute lymphoblastic leukemia (ALL) have arisen from adaptation of ALL treatment in children. Applications of pediatric schedules to adults have thus been used, providing increased drug intensity at several stages of treatment with mainly larger cumulative doses of corticosteroids, vincristine, and l-asparaginase. With current regimens, the complete remission (CR) rate reaches 80–90% and survival rates at 5 years range from 67 to 78% which compare favorably to rates between 34 and 41% observed with the former protocols [Citation1]. Despite improvements in upfront treatment, the outcome in patients with refractory/relapsed (R/R) ALL remains dismal. Salvage chemotherapy results are modest in this setting [Citation2–4]. Only 30–40% of adults achieve a second CR and 10–20% in further salvages. The median overall survival (OS) ranges from 4.5 to 8.4 months and 5-year survival rates are just 7–10%. At this stage, only allogeneic stem cell transplantation (SCT) offers a chance of long-term survival. However, only few patients can be bridged to allogeneic SCT [Citation2,Citation3]. There is therefore an unmet need for novel agents in the treatment of patients with R/R ALL to improve outcome [Citation5].
Lenalidomide is a second-generation immunomodulatory compound which, either as single agent or in combination with other drugs, has shown interesting results as a therapeutic option for patients with R/R multiple myeloma or other hematological malignancies. Lenalidomide has been approved in combination with dexamethasone for the treatment of multiple myeloma [Citation6,Citation7] and in combination with rituximab for the treatment of mantle-cell lymphoma [Citation8]. Numerous clinical trials, as a single agent or in combination therapy, are currently ongoing in B-cell non-Hodgkin lymphoma, and it has clearly demonstrated anti-leukemic activity in chronic lymphocytic leukemia patients [Citation9,Citation10]. Lenalidomide displays pleiotropic anti-tumor effects, including stimulation of T-cell and natural killer-cell expansion, inhibition of tumor-associated angiogenesis and lymphangiogenesis, and induction of apoptosis through the downregulation of cyclin D1 [Citation11–16].
Based on these clinical and biological data in B-cell malignancies, we hypothesized that management of B-cell lineage ALL with this biologic agent might offer patients a potential disease control with a favorable side effect profile relative to chemotherapy approaches. We therefore conducted an “open-label” multicenter, phase 1/2 study to evaluate the safety and efficacy of the combination of lenalidomide and dexamethasone, as used in multiple myeloma, in the treatment of adult patients with R/R B-cell lineage ALL.
Patients and methods
Patient eligibility
The study (EUDRACT # 2009-009372-13) was conducted at four French centers of the Rhône-Alpes Auvergne region. The study enrolled adult patients (≥18 years old) with documented B-cell lineage ALL, who were refractory or had failed to at least two treatment regimens (bone marrow transplantation could be considered as one line of treatment). Inclusion of patients with Philadelphia chromosome-positive (Ph+) ALL was authorized in the presence of a T315I mutation and absence of investigational trial targeting this abnormality. Other requirements for enrollment included an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2, a life expectancy of at least 3 months, an adequate liver function (AST and/or ALT not > 3 times the upper limits of the normal range), an adequate renal function (calculated creatinine clearance >50 ml/minute), the absence of uncontrolled infection, the absence of central nervous system involvement, the absence of known neoplastic malignancy during the past 5 years, no grade ≥ 2 peripheral neuropathy, or a contraindication for dexamethasone. To avoid potential embryo-fetal toxicity, patients had to agree with the Risk Evaluation and Mitigation Strategy program (REMS) and to use an effective method of contraception during the study.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice and applicable regulations. Written informed consent was obtained from each patient before any study procedure was undertaken. The Institutional Review Board at Saint-Etienne University Hospital approved the study and the contents of the informed consent document.
Study design
The dose and schedule of lenalidomide and dexamethasone selected for this current study was based on the one used in multiple myeloma. Lenalidomide was given orally at a dose of 25 mg/day on days 1 through 21 of 28-day cycles, in combination with dexamethasone given weekly at 40 mg per os once daily on days 1, 8, 15, 22 of each 28-day cycle. Cycles were continued until disease progression, development of unacceptable adverse effects, or withdrawal from the study.
Patients with a prior history of deep-vein thrombosis or pulmonary embolism received prophylactic anticoagulation with a low-molecular-weight heparin therapy for at least the first 4 months of study participation. Patients who had not experienced deep-vein thrombosis or pulmonary embolism received low-dose aspirin or other prophylactic antithrombotic treatment at the discretion of the investigator. Tumor lysis syndrome prophylaxis consisted of hyperhydratation and hypouricemic treatment. Prophylactic growth factors, such as granulocyte colony-stimulating factor, were empirically administered because of the hematological toxicity of lenalidomide. Biphosphonates and other supportive therapies, such as prophylaxis against herpes zoster, were allowed at the investigator’s discretion.
Efficacy and safety assessments
Primary endpoints were overall response rate (ORR) (defined as CR, complete remission without complete platelet count recovery [CRp] or partial response [PR]) and safety (type, frequency, and severity of adverse events, and their relationship to study drug). The initial response assessment was performed at the end of cycle 1. Patients were eligible to receive additional cycles of treatment if a response to treatment (CR, CRp, or PR) or sufficient clinical activity (a ≥50% decrease in leukemic cell counts in the peripheral blood and/or bone marrow or evidence of clinical response in the lymph nodes or mediastinal mass) were observed. Patients achieving CR and having an identified HLA compatible donor could undergo allogeneic SCT. CR was defined as no evidence of circulating blasts or extramedullary disease, a bone marrow with ≤5% blasts, and recovery of peripheral counts (platelets ≥100 G/l and absolute neutrophil count ≥1 G/l). CRp was defined as meeting all criteria for CR except for recovery of platelet counts to ≥100 G/l. PR was defined as complete disappearance of circulating blasts, a bone marrow with >5 to ≤25% blasts, and appearance of normal progenitor cells or a bone marrow with ≤5% blasts that did not qualify for CR or CRp. The severity of adverse events was graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE version 3.0 Toxicity Scale). The intent-to-treat (ITT) population comprised all enrolled patients regardless of whether they received the study drug. The safety population included all patients who met the protocol requirements and received at least one dose of the study drug. The efficacy evaluable (EE) population included patients who met the protocol requirements and were evaluated after receiving at least one cycle of the study drug.
Secondary endpoints included: time to response, duration of response, and overall survival (OS) (calculated from the beginning of salvage therapy to the time of death).
Quality of life analysis
Health-related quality of life was measured with the use of the EORTC-QLQ-C30 questionnaire, which includes a general questionnaire that assesses physical well-being, emotional well-being, social well-being, and functional well-being – with higher scores on each component indicating higher quality of life. The assessment was performed at baseline, and after each cycle.
Statistical analysis
A Simon’s two-stage design was initially planned [Citation17] with accrual of 13 patients in the first stage and 14 additional patients in the second stage if at least one response was initially observed. Treatment could be considered efficient if at least four responses were observed (α = 0.05, power = 0.8). Actually, because of a smaller number of patients leading to premature arrest of inclusions, statistics were mainly descriptive. Exact binomial 95% confidence intervals (CIs) were reported for proportions. Kaplan–Meier methodology was used to estimate distribution of OS (time of treatment initiation to death).
Results
Patient characteristics
Between February 2010 and August 2013, 10 patients were enrolled in the study. shows patients’ baseline characteristics and prior therapies. One patient with a relapsed T-cell lineage ALL and one patient with a tyrosine kinase inhibitor (TKI) refractory Ph+ ALL, but without T315I mutation, were inappropriately included and were not considered for the EE and the safety population analyses. However, they were considered for the ITT analysis. Only eight patients were therefore included in the safety population analysis and only seven in the EE population analysis since one patient did not complete the first cycle because he died from hemorrhagic complication on day 12 ().
Figure 1. CONSORT flow diagram. Abbreviations: EE: efficacy evaluable; ITT: intention-to-treat; PD: progressive disease; PR: partial remission; pt(s): patients; SD: stable disease.
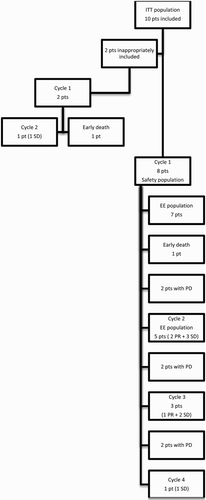
Table 1. Patients’ baseline characteristics, prior therapies, and outcomes.
The median age of the population analyzed for safety was 63 years (range, 46–74 years). There were two females and six males. Two patients were treated for relapsed Ph+ ALL (with T315I mutation) and six patients for relapsed B-cell lineage ALL. At the time of inclusion, all patients had already received one or two prior lines of therapy; none underwent SCT either during first line or in rescue after the first relapse. After first-line therapy, only two patients obtained a long first CR: >2 years and >5 years, respectively. After second-line therapy, only one patient achieved CR and kept this status for a very short period of time (14 days). The mean white blood cell (WBC) count at inclusion was 8.7 G/l with a mean peripheral blast cell count of 4.3 G/l.
Efficacy
Evaluable patients received a median of two treatment cycles (range, one to four cycles). Patient numbers were small for the analyses, which should be interpreted in this context. The ORR was 28.6% (95% CI, 0–62.2%) with two patients achieving partial response after one or two courses. No CR was observed. Three other patients who had shown sufficient treatment activity (with a decrease in blast cell count ≥50% as compared to baseline in peripheral blood) pursued additional treatment cycles. Response duration was short (three cycles) and no patient could undergo allogeneic SCT procedure. Median OS was 92 days (range, 43–133 days). All evaluable patients have died because of progressive disease.
Regarding ITT population analysis, ORR was 20% (95% CI, 0–44.8%) with only two PR. Median OS was 81 days (range, 12–133 days).
Adverse events
Treatment was associated with toxic effects that have been reported previously with these agents. Treatment was mainly ambulatory. No patient experienced tumor lysis syndrome or thrombotic complications. In the safety population, there was no death due to treatment-related adverse events. One patient died early (day 12) from hemorrhage before completing cycle 1. This was related to severe refractory thrombocytopenia and not attributed to the studied drug combination. The most common grade 3 or 4 adverse events were hematological adverse events including neutropenia, thrombocytopenia and anemia, and were manageable with standard interventions. Median durations of neutropenia <0.5 G/l and platelets <20 G/l were of 15 days and 13 days, respectively. Grade 3 or 4 non-hematologic adverse events are listed in . Grade 3 infections requiring hospitalization – including two cases of pneumonia and three cases of bacteremia (one Bacillus gram-negative, one Listeria monocytogenes, one Pneumoccocus) – were reported during cycles. All resolved with the administration of antibiotics and supportive care.
Table 2. Hematological and non-hematological grade 3 or 4 adverse events in the safety population (16 cycles).
Quality-of-life assessment
The completion rate of the EORTC-QLQ-C30 questionnaires was 50% at any given time point. Quality-of-life scores remained stable throughout treatment cycles. Global health status scoring was comparable between the beginning and the end of each cycle.
Discussion
Treatment of refractory/relapsed adult ALL remains a considerable challenge. Despite promising data in B cell malignancies, single agent lenalidomide combined with dexamethasone was ineffective to really control the disease in R/R adult ALL. Of note, all patients enrolled in this study had a very poor prognosis with first CR duration of less than 1 year for most of them and failure to achieve CR after first relapse. Nevertheless two PR were achieved: one after one cycle and one after two cycles in patients who were refractory to the prior line of treatment. To our knowledge, very few data are available about lenalidomide in the treatment of ALL. A phase 1 trial of lenalidomide in acute leukemia, which enrolled 31 adults with acute myeloid leukemia and four adults with ALL, did not observe any response in ALL patients [Citation18]. A recent Japanese phase 1 dose-escalation study enrolled nine patients with relapsed T-cell aggressive leukemia/lymphoma [Citation19]. The optimal dose was identified as 25 mg/day given continuously which is currently being tested in a phase 2 study. T-cell lineage ALL was not included in our trial, but the patient remained with a stable disease for two cycles.
The major limitation of this trial was the small number of included patients, which was much lower than the number initially planned leading to a premature ending of the trial before validation of our hypothesis regarding treatment efficacy in accordance to Simon’s two-stage design plan. This was mainly due to the concurrent development of trials based on monoclonal antibody therapy.
Despite high rate of grade 3/4 hematologic toxicity, the combination of lenalidomide with dexamethasone appeared feasible as an ambulatory treatment and toxicities observed were easily manageable as compared to those potentially observed with polychemotherapy regimens. These treatment-related side effects were expected as we used the maximum tolerated dose of lenalidomide (25 mg daily). There were no treatment-induced deaths and the stable trajectory of health-related quality of life reflected the overall absence of cumulative treatment-related toxic effects. Although only two PRs were observed in our short series of very high-risk ALL patients, further studies testing lenalidomide in combination with cytotoxic agents or immune-based therapies should certainly be explored. In mantle-cell lymphoma, combinations of lenalidomide with rituximab were shown to be highly promising with high response rates [Citation20,Citation21]. In relapsed or refractory aggressive lymphoma, addition of lenalidomide to polychemotherapy yielded encouraging results with high ORR [Citation22,Citation23] leading to ongoing large randomized trials to confirm these results and determine the optimal position of lenalidomide in the association.
In B-cell lineage ALL, new therapeutic strategies are becoming available with the development of immune therapies that recruit (bispecific T cell engager) or modify (chimeric antigen receptor T cells) patient’s own T cells to fight leukemic cells [Citation24,Citation25]. These new approaches lead us to predict that, in a few years, ALL therapy might be based heavily on non-chemotherapeutic approaches. In this setting, given lenalidomide’s unique immune stimulatory properties and its oral bioavailability, there is potential for further development of this compound in concomitant or sequential combination regimens with these new antitumor agents. However, the current challenge in R/R ALL patients is to propose an effective and safe salvage regimen that could serve as a bridge to allogeneic SCT. Because of recent encouraging data with combination therapies involving lenalidomide in other B-cell malignancies, further investigations should focus on better determining lenalidomide mechanism of action in ALL and testing various combinations, in order to optimize its efficacy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr E. Tavernier-Tardy is responsible for acute leukemia treatments in the hematology department in Institut de Cancérologie de la Loire. She is a member of the French Society of hematology and GRAAL group (Group of research for Adult Acute Leukemia).
Dr J. Cornillon is responsible for bone marrow transplantation in the hematology department in Institut de Cancérologie de la Loire. He is a member of the French Society of Bone Marrow transplantation (SFGM-TC).
Dr C. Molucon-Chabrot is working in the hematology unit in the University Hospital in Clermont-Ferrand.
Pr J. Y. Cahn is the head of the hematology department in the University Hospital in Grenoble.
F. Tinquaut is working as a statistician in Institut de Cancérologie de la Loire.
Dr A. Bourmaud ensures that the methodology of clinical trials is conducted in Institut de Cancérologie de la Loire. She has expertise in Public Health, Oncology, and Epidemiology.
Pr D. Guyotat is the head of the hematology department in Institut de Cancérologie de la Loire.
Dr X. Thomas is responsible for acute leukemia treatments in the hematology department in University Hospital in Lyon. He is a member of the ALFA group (acute myeloid leukemia) and GRAAAL group (Group of research for Adult Acute Leukemia).
ORCID
A. Bourmaud http://orcid.org/0000-0002-2228-6471
References
- Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–1914. doi: 10.1038/sj.leu.2404824
- Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–2041. doi: 10.1182/blood-2011-12-399287
- Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192
- Frey NV, Luger SM. How I treat adults with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Blood. 2015;126:589–596. doi: 10.1182/blood-2014-09-551937
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–917. doi: 10.1056/NEJMoa1402551
- Goy A, Sinha R, Williams ME, et al. Single agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;10:3688–3695. doi: 10.1200/JCO.2013.49.2835
- Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401
- Chen CI, Paul H, Wang T, et al. Long-term follow-up of a phase 2 trial of single agent lenalidomide in previously untreated patients with chronic lymphocytic leukaemia. Br J Haematol. 2014;165:731–733. doi: 10.1111/bjh.12785
- Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56–63. doi: 10.1016/j.mvr.2005.01.002
- Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386.
- Hayashi T, Hideshima T, Akiyama M, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical applications. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x
- Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323
- Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45.
- Moros A, Bustany S, Cahu J, et al. Antitumoral activity of lenalidomide in in vitro and in vivo models of mantle cell lymphoma involves the destabilization of cyclin D1/p27K1P1 complexes. Clin Cancer Res. 2014;20:393–403. doi: 10.1158/1078-0432.CCR-13-1569
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9
- Blum W, Klisovic RB, Becker H, et al. Dose escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol. 2010;28:4919–4925. doi: 10.1200/JCO.2010.30.3339
- Ogura M, Imaizumi Y, Uike N, et al. Lenalidomide in relapsed adult T-cell leukaemia-lymphoma or peripheral T-cell lymphoma (ATLL-001): a phase 1, multicentre, dose-escalation study. Lancet Haematol. 2016;3:e107–e118. doi: 10.1016/S2352-3026(15)00284-7
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–723. doi: 10.1016/S1470-2045(12)70200-0
- Ruan J, Martin P, Shah B, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N Engl J Med. 2015;373:1835–1844. doi: 10.1056/NEJMoa1505237
- Martin A, Redondo AM, Dlouhy I, et al. Lenalidomide in combination with R-ESHAP in patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 1b study from GETALMO group. Br J Haematol. 2016;173:245–252. doi: 10.1111/bjh.13945
- Feldman T, Mato AR, Chow KF, et al. Addition of lenalidomide to rituximab, ifosfamide, carboplatin, etoposide (RICER) in first-relapse/primary refractory diffuse large B-cell lymphoma. Br J Haematol. 2014;166:77–83. doi: 10.1111/bjh.12846
- Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicenter, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222
- Rousselot P, Coudé MM, Gökbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia-chromosome positive ALL. Blood. 2016; [ in press].
- Schwartz P, Hunault-Berger M, Chevallier P, et al. French results of the EWALL chemotherapy backbone in older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia. A GRAALL report. Haematologica. 2013;98(Suppl.1):463.
- Domenech C, Thomas X, Chabaud S, et al. L-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomised trial. Br J Haematol. 2011;153:58–65. doi: 10.1111/j.1365-2141.2011.08588.x
- Hunault-Berger M, Leguay T, Huguet F, et al. A phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study. Am J Hematol. 2015;90:811–818. doi: 10.1002/ajh.24093
- Huguet F, Leguay T, Raffoux E, et al. Clofarabine for the treatment of adult acute lymphoid leukemia: the group for research on adult acute lymphoblastic leukemia intergroup. Leuk Lymphoma. 2015;56:847–857. doi: 10.3109/10428194.2014.887708
