ABSTRACT
Objective: To evaluate the association between endothelial dysfunction and otoneurological symptoms and vaso-occlusive phenomena in children with sickle cell disease (SCD).
Methods: Cross-sectional study with 54 children, aged between 6 and19 years of age, of whom 28 had genotype SS and 26 apparently healthy (AA genotype) whose parents or guardians, or the children themselves, filled out a questionnaire designed to assess their otoneurological symptoms. All the individuals were submitted assessment of endothelial function by flow-mediated dilation (FMD) percentage with reactive hyperemia of brachial artery Doppler.
Results: Otoneurological symptoms (tinnitus and/or vertigo) predominated in the SCD group (46.4 vs. 15.4%; p = 0.006). A negative correlation was observed between FMD percentage and time of evolution of vertigo SCD (r = −0.432; p = 0.022) and the linear regression analysis demonstrated that for every reduction in FMD percentage there was an increase in time of evolution of vertigo of 1.79 months (β = −1.79; p = 0.022). The positive correlation between episodes of painful crisis and time of evolution of vertigo (r = 0.3; p = 0.04).
Discussion: The presence of vascular endothelial damage in the labyrinthine artery in patients with SCD is capable of compromising the semicircular canals, shown by clinical expression of otoneurological symptoms, such as vertigo. In the present study, an association was observed between endothelial dysfunction with otoneurological symptoms and otoneurological symptoms and vaso-occlusive phenomena in SCD.
Introduction
The clinical presentation of sickle cell disease (SCD) is characterized by hemoglobin polymerization S and consequently vaso-occlusive phenomena [Citation1], it is manifested clinically by episodes of painful crisis. Studies have demonstrated that these alterations are capable of generating otoneurological symptoms [Citation2–6] such as sensorineural hearing loss (SNHL), tinnitus, and vertigo [Citation5,Citation7]. The literature has demonstrated that its clinical presentation can be related to endothelial dysfunction [Citation8].
The reduction of flow-mediated dilatation (FMD), secondary to reduction of nitric oxide (NO) release, followed by the increase in wall shear stress has been considered an early marker of vascular alterations in SCD patients [Citation9]. Endothelial function evaluation by ultrasonography is a non-invasive method for early detection of changes in the brachial artery wall functionality [Citation10,Citation11].
Therefore, the objective of the present study is to evaluate the association between otoneurological symptoms and endothelial dysfunction and vaso-occlusive phenomena in children with SCD.
Methods
Selection and description of participants
This was a cross-sectional, analytical study, with a non-probabilistic, sequential sample, in which 54 children and adolescents individuals in the age-range ≥6 and ≤18 years. Of these, 28 were diagnosed with SCD with hemoglobin SS (HbSS), composed the case group, and were patients originating from the University Hospital Professor Edgar Santos of the Federal University of Bahia (UFBA). The Control Group of 26 apparently healthy subjects whose hemoglobin genotype was AA, originated from the School of Medicine of Bahia, Terreiro de Jesus of the UFBA. These two groups were matched for age, during the period from January through July 2015.
This study was approved by the Research Ethics Committee of the Bahiana School of Medicine and Public Health (No. 33705714.3.0000.5544) and informed consent was obtained from all the children’s parents or guardians, and also from the children who were mature enough to understand the study goals.
The study was designed with 90% power to detect a meaningful magnitude of difference superior to two standard deviations of 0.25 in the outcome (reduction in FMD percentage) between the case and the control groups. Considering a type I error of 0.05, and a type II error of 0.10, 13 participants per group were required. However, in order to achieve the study objectives and to include the possibility of a 10% loss, the final sample size was increased to 26 in each group.
Inclusion criteria for SCD group were: age between 6 and 18 with a diagnosis confirmed by DNA sequence, hemoglobin electrophoresis and/or high performance of liquid chromatography. Inclusion criteria for the control group were: age between 6 and 18 and being apparently healthy.
Exclusion criteria for the SCD group were: the presence of other hemoglobinopathies; associated genetic syndromes; obesity; diabetes; hypercholesterolemia; blood transfusion in the last 90 days; acute events in fewer than 30 days previously; stroke. Exclusion criteria for the control group were: presence of infection in the last 30 days; neurologic disease; heart disease, diabetes, genetic syndromes, lung disease, obesity, or hypercholesterolemia.
Technical information
Age was measured by complete years from the date of birth. Race was self-reported according to the official terms used in the demographic census of the Brazilian population. Using skin color as a parameter, the reported categories were white, black, and mixed race, and clinical data were obtained from patient records as hemoglobin; fetal hemoglobin dosage, hydroxyurea use and time of use, presence of episode of painful crisis (vaso-occlusive phenomena) in the last 12 months and number of episodes of painful crisis) in the last 12 months to SCD group.
A questionnaire adapted from the study of Silva [Citation12] was used for assessment otoneurological symptoms. The answers were given by the parents or guardians, as well as by the patients with SCD and control group: “Does your child presented tinnitus?;” “For how many months has he or she presented tinnitus?” (time of evolution of tinnitus); “Does your child have vertigo (rotating dizziness)?;” “For how many months has he or she presented vertigo?” (time of evolution of vertigo); “Are the episodes of vertigo associated with nausea?” The otoneurological symptoms were determined by the presence of tinnitus and/or vertigo, thus as time of evolution of otoneurological symptoms as the larger time of evolution of tinnitus or time of evolution of vertigo.
The questions were applied by two calibrated examiners, who were blind to the answers obtained. They carried out this research step in distinct time intervals, with the goal of minimizing the possible failures related to the vertigo and tinnitus symptoms. The answers were considered positive when there was agreement between the two examiners.
Information pertaining the weight and height were obtained by clinical assessment using a Welmy® model R-110 scale with a stadiometer and the body mass index (BMI) was obtained in accordance with the “Quetelet” index weight quotient for the height squared (kg/m2). The classification according WHO (2007) [Citation13].
A protocol established for ultrasonography evaluation of the brachial artery was used to assess endothelial function [Citation14,Citation15]. The exams were performed in the Cardiovascular Research Laboratory of the Bahiana School of Medicine and Public Health (EBMSP). The equipment used was a VIVID 3 ultrasound system (General Electric Company – Israel), with a multifrequency ultrasonic transducer ranging from 7 to 12 MHz.
Endothelial function was evaluated by means of FMD with reactive hyperemia. The exams were performed in patients who had fasted for 4hours, after 30 minute rest, with room temperature between 20 and 25°C. All the exams were performed in the morning period to avoid circadian variations. The patients were examined in dorsal decubitus, with the arm positioned ergonomically; the electrocardiogram was synchronized and the heart rate was checked. The mercury column sphygmomanometer cuff functioned as a pneumatic tourniquet around the right arm above the elbow flexure. The diameter of the brachial artery was measured in a longitudinal section (2–15 cm above the elbow) with a high-resolution vascular ultrasound instrument, and then identified in light, at the anterior wall media-adventitial interface (“M line”) and the posterior wall intima–lumen interface. The Doppler sample was positioned at a 60° angle; with gray scale control adequacy, depth, filter and Doppler scale. The flow increase was induced by cuff insufflation around the arm to 250 mmHg, for 4 minutes, with continuous monitoring of the artery image, followed by cuff disinflation, leading to reactive hyperemia. The first five flows (Doppler maximum velocity measurement) and artery diameter were monitored for 120 seconds (for measuring the diameter at the end 60 seconds). An experienced vascular ultrasonographer, blind to the patient’s diagnosis, performed and analyzed all images recorded in a high-quality computer. The FMD was then calculated as the percent change in diameter compared with baseline resting diameters.
Statistics
All statistical evaluations and operations were performed using the computer software application Statistical Package for Social Sciences (SPSS) version 21 to analyze the real agreement between the two examiners about the answers obtained from the questionnaire the statistical Kappa Test was applied.
Quantitative variables were reported as the means ± standard deviation or median and interquartile range and categorical variables were reported as simple and relative frequencies. In this cross-sectional study, the chi-square test was used to verify the association between qualitative variables; the Mann–Whitney or t-Student test for the association between qualitative and continuous variables; the Pearson and Spearman tests for correlation between two continuous variables and linear regression analysis.
Results
Fifty four children aged between 6 and 18 years of age were evaluated; 28 with SCD, while 26 were controls with hemoglobin genotype AA. shows the sociodemographic and clinical profile of children and adolescents in SCD group and control group.
Table 1. Sociodemographic and clinical profile of SCD group and control group individuals.
Participants with SCD presented low average fetal hemoglobin of 7.8 ± 5.8 g/dl, 28 (100%) presented episodes of painful crisis in the last 12 months and the median of episodes of painful crisis in the last 12 months was 2 (0.25–3.75), 14(50%) hydroxyurea use and the median of time of use of 3.32 ± 1.92 years.
FMD percentage was significantly decreased in SCD group compared with controls (11.6 ± 5.58 vs. 19.31 ± 9.34%, p = 0.008).
Agreement between the two examiners was considered satisfactory with the respective kappa values that were obtained with regard to the questions: “Does your child presented tinnitus” – kappa = 0.82; “For how many months has he or she presented tinnitus?” (time of evolution of tinnitus) – kappa = 0.68; “Does your child have vertigo (rotating dizziness)?” – kappa = 1; “For how many months has he or she presented vertigo?” (time of evolution of vertigo); – kappa = 0.79; “Are the episodes of vertigo associated with nausea?” – Kappa = 1;
Otoneurological symptoms (tinnitus and/or vertigo) predominated in the SCD when compared with the control group (46.4 vs. 15.4%; p = 0.006). In the SCD group, 42.9% presented tinnitus and 46.4 presented vertigo. shows the association between the presence of otoneurological symptoms in the SCD and control groups.
Table 2. Comparison of otoneurological profile between SCD group and control group individuals.
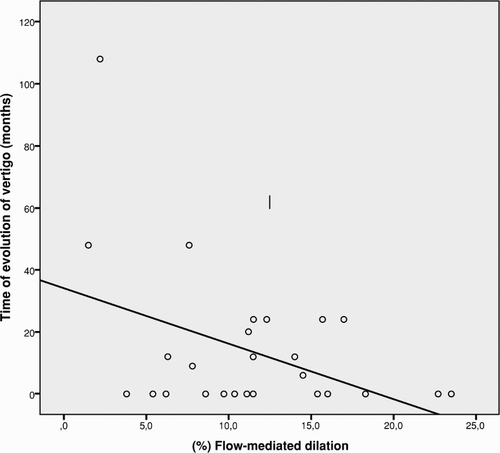
The Pearson correlation analysis demonstrated a significant inverse correlation between FMD percentage and time of evolution of vertigo in SCD (r = −0.4; p = 0.02).
The result of the linear regression analysis between FMD percentage and the time of evolution of vertigo () demonstrated that variation in the FMD percentage influence the time of evolution of vertigo. The linear regression coefficient indicated that for each percentage reduction in FMD there was a rise in the time of evolution of vertigo by 1.79 months (β = 1.79; p = 0.02).
Figure 1. The linear regression analysis between flow-mediated dilation percentage and the time of evolution of vertigo expressed in months for 28 participants with SCD (p = −0.432; p = 0.022; β = −1.79).
The Spearman correlation analysis showed a positive correlation between the number of episodes of painful crisis and time of evolution of vertigo (r = 0.3; p = 0.04).
Discussion
To the best of our knowledge, this is the first study that showed an association between endothelial dysfunction and otoneurological symptoms by means of the inverse correlation in which the lower flow-mediated dilation (FMD), the higher the time of evolution of vertigo in children and adolescents with SCD and the impact of the association demonstrated in the linear regression analysis
The presence of endothelial dysfunction in SCD occurs due to wall shear stress, sickle cell, and endothelial cell adherence and interaction, low O2 tension, and high viscosity. This cascade of events leads to vessel occlusion and perfusion damage that induces inflammation and endothelial lesion, lower production of nitric oxide (NO) – the main vasodilator – and increase in expression of vascular cell adhesion molecule 1 (VCAM-1) by endothelial cells [Citation11,Citation16,Citation17]. The literature points to reduction in NO, one of the markers of endothelial dysfunction in the internal ear structures (cochlea and semicircular canals) for otoneurological symptoms in patients with acute idiopathic tinnitus [Citation18]. While VCAM-1 has been considered a marker of endothelial dysfunction in SNHL [Citation19]. When endothelial dysfunction is present in the auditory system, it is capable of causing circulatory disturbances, as the labyrinthine artery is the only blood supply to this system. Impairment of perfusion by this artery is capable of generating sudden hearing loss, alterations in outer hair cells which are the sensory cells of audition, in the semicircular canals (SC) responsible for corporal balance, as well as in the VIII cranial nerve (vestibulocochlear nerve) with subsequent sensorineural hearing loss and tinnitus [Citation8]. Thus, the present study suggests that the presence of vascular endothelial damage in the labyrinthine artery in patients with SCD is capable of compromising the semicircular canals, clinically expressed as otoneurological symptoms such as vertigo. In addition, it has been observed that longer periods of vertigo involvement were associated with a lower percentage of vasodilation, indicating worsening in endothelial function.
In this research, endothelial dysfunction was also observed by the lower FMD percentage in the SCD group when compared with the control group. The study of De Montalembert et al. [Citation10] achieved similar results, when evaluating FMD in children with SCD (HbSS and Thalassemia), by the reduction in brachial artery FMD shown by means of the non-invasive ultrasound technique.
The presence of otoneurological symptoms was greater in SCD group than in the control group. Piltcher et al. [Citation20] conducted a case–control study that demonstrated that the frequency of otoneurological symptoms (SNHL, tinnitus, and vertigo) was also greater in SCD group when compared with the control group (50 vs. 10.7%; p = 0.01).
In the present study, we also observed an association between the number of painful crises in the last 12 months and the time of evolution of vertigo. When a vaso-occlusive crisis occurs in the inner ear, it can lead to otoneurological symptoms as a result of ischemic damage [Citation2,Citation5,Citation6]. In SCD, the increase in blood viscosity may be related to hemoglobin crystallization, consequently leading to micro vascular ischemia in the cochlea and stria vascularis. These alterations lead to Organ of Corti hypoxia and reduction in blood flow to the neurons responsible for the electrical impulses along the central auditory pathway, resulting in otoneurological symptoms [Citation2–6].
Another important aspect of this study was the assessment of endothelial function by a method easily performed by a trained professional, allowing early inferences about its association with otoneurological symptoms, especially sensorineural hearing loss related to learning problems of school-age children. In addition, the importance of valuing children’s complaints is noteworthy, as well as the inclusion of routine hearing evaluation in pediatric and hematologic follow-up examinations.
This study had some methodological limitations. It was a study conducted by means of interviews, which could be biased by the participants’ memory as regards the presence and time of evolution of otoneurological symptoms. In addition, the children might have had difficulties with describing the symptoms. However, these limitations are inherent to questionnaires that involve the identification of symptoms and clinical manifestations of chronic diseases.
Therefore, this study allowed the authors to conclude that there was an association between endothelial dysfunction and otoneurological symptoms and the clinical manifestations of vaso-occlusive phenomena in children and adolescents with SCD. This suggested that the endothelial aggression may be considered a trigger for vascular involvement in auditory manifestations in this population.
Acknowledgements
This article is part of Mara Renata Rissatto Lago PhD thesis of Bahiana School of Medicine and Public Health PostGraduate Course.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mara Renata Rissatto-Lago MD, Msc. PhD Student of the Postgraduate Course in Medicine and Human Health of the Bahiana School of Medicine and Public Health. Speech-language pathologist and Assistant Professor of the Department of the Life Science, State University of Bahia.
Cristina Salles MD, PhD. Postdoctoral Student of the Postgraduate Course in Medicine and Human Health of the Bahiana School of Medicine and Public Health (EBMSP). Adjunct professor of the EBMSP. Preceptor and Otorhinolaryngology of the Hospital Universitário Professor Edgar Santos of UFBA.
Fernando Gesteira Campos de Pinho Undergraduate Student of Medicine of the Bahiana School of Medicine and Public Health.
Isa Menezes Lyra MD, PhD. Full IV professor at University Salvador-Laureate International Universities and Hematologist at UFBA.
Regina Terse-Ramos MD, PhD. Adjunct Professor of the Department of Pediatrics of the School of Medicine of Bahia at UFBA. Collaborating Professor of the Postgraduate Course in Medicine and Health of Hospital Universitário Professor Edgar Santos of UFBA.
Rozana Teixeira MD, Msc. PhD Student of the Postgraduate Course in Medicine and Human Health of Bahiana School of Medicine and Public Health (EBMSP). Assistant Professor of the EBMSP and Auxiliary Professor of the Department of Pediatrics at UFBA.
Ana Marice Ladeia MD, PhD. Adjunct Professor of Bahiana School of Medicine and Public Health (EBMSP), Coordinator of the Postgraduate Course in Medicine and Human Health of EBMSP. Cardiologist of the Hospital Roberto Santos – Health Department of the State of Bahia. Coordinator of projects funded by national research agencies, studying the following topics: atherosclerosis, endothelial function and inflammation, dyslipidemia, sickle cell disease, diabetes, and obesity. Research Grant for Scientific Productivity of the National Council of Technological and Scientific Development-CNPQ. Reviewer of national and international journals.
ORCID
Regina Terse-Ramos http://orcid.org/0000-0002-5277-3609
Rozana Teixeira http://orcid.org/0000-0001-7693-3817
Ana Marice Ladeia http://orcid.org/0000-0002-2235-7401
Additional information
Funding
References
- Thornton JB, Sams DR. Preanesthesia transfusion and sickle cell anemia patients: case report and controversies. Spec Care Dentist. 1993;13(6):254–257. doi: 10.1111/j.1754-4505.1993.tb01478.x
- Burch-Sims GP, Matlock VR. Hearing loss and auditory function in sickle cell disease. J Commun Disord. 2005;38(4 Spec. iss.):321–329. doi: 10.1016/j.jcomdis.2005.02.007
- Mgbor N, Emodi I. Sensorineural hearing loss in Nigerian children with sickle cell disease. Int J Pediatr Otorhinolaryngol. 2004;68(11):1413–1416. doi: 10.1016/j.ijporl.2004.05.009
- Downs CR, Stuart A, Holbert D. Distortion product otoacoustic emissions in normal-hearing children with homozygous sickle cell disease. J Commun Disord. 2000;33(2):111–127; quiz 128–129. doi: 10.1016/S0021-9924(99)00027-1
- Saito N, Watanabe M, Liao J, et al. Clinical and radiologic findings of inner ear involvement in sickle cell disease. Am J Neuroradiol. 2011;32(11):2160–2164. doi: 10.3174/ajnr.A2720
- Silva LP, Nova CV, Lucena R. Sickle cell anemia and hearing loss among children and youngsters: literature review. Braz J Otorhinolaryngol. 2012;78(1):126–131. doi: 10.1590/S1808-86942012000100020
- Whitehead RE, MacDonald CB, Melhem ER, et al. Spontaneous labyrinthine hemorrhage in sickle cell disease. Am J Neuroradiol. 1998;19(8):1437–1440.
- Otol A. Sensorineural hearing loss and endothelial dysfunction due to oxidative stress: is there a connection? Andre Ciorba, Milvia Chicca, Chiara Bianchini, Claudia Aimoni, Antonio Pastore. 2012;(l):16–20.
- Belhassen L, Pelle G, Sediame S, et al. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood. 2001;97(6):1584–1589. doi: 10.1182/blood.V97.6.1584
- De Montalembert M, Aggoun Y, Niakate A, et al. Endothelial-dependent vasodilation is impaired in children with sickle cell disease. Haematologica. 2007;92(12):1709–1710. doi: 10.3324/haematol.11253
- Zawar SD, Vyawahare MA, Nerkar M, et al. Non-invasive detection of endothelial dysfunction in sickle cell disease by Doppler ultrasonography. J Assoc Physicians India. 2005;53:677–680.
- De Castro Silva IM. Avaliação eletrofisiológica da audição em portadores de doença falciforme. Brasília: Universidade de Brasília; 2009.
- Brasil. Ministério da Saude. Incorporação da curvas de crescimento da Organização Mundial da Saúde de 2006 e 2007 no SISVAN. 2007;1–38.
- Moens AL. Flow-mediated vasodilation. CHEST J [Internet]. 2005;127(6):2254. Available from: http://journal.publications.chestnet.org/article.aspx?doi = 10.1378/chest.127.6.2254
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-F
- Gualandro SFM, Fonseca GHH, Gualandro DM. Complicações cardiopulmonares das doenças falciformes. Rev Bras Hematol Hemoter. 2007;29(3):291–298. doi: 10.1590/S1516-84842007000300020
- Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Curr Opin Hematol. 2003;10(2):99–107. doi: 10.1097/00062752-200303000-00001
- Neri S, Signorelli S, Pulvirenti D, et al. Oxidative stress, nitric oxide, endothelial dysfunction and tinnitus. Free Radic Res. 2006;40(6):615–618. doi: 10.1080/10715760600623825
- Quaranta N, Ramunni A, Brescia P, et al. Soluble intercellular adhesion molecule 1 and soluble vascular cell adhesion molecule 1 in sudden hearing loss. Otol Neurotol. 2008;29(4):470–474. doi: 10.1097/MAO.0b013e318170b650
- Piltcher O, Cigana L, Friedriech J, et al. Sensorineural hearing loss among sickle cell disease patients from southern Brazil. Am J Otolaryngol. 2000;21(2):75–79. doi: 10.1016/S0196-0709(00)85001-2
