ABSTRACT
Purpose: Extranodal natural killer/T-cell lymphoma (ENKTL), nasal-type, is a distinct subtype of non-Hodgkin lymphoma. ENKTL is sensitive to radiotherapy, but the prognosis is poorer than those of other types of early stage lymphoma. To date, optimal treatment strategies for patients with early stage ENKTL have not been fully defined.
Methods: We retrospectively investigated the efficacy and safety of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) combined with different dose radiotherapy (RT) in the treatment of 35 newly diagnosed, stage IE to IIE ENKTL patients at our institution from October 2011 to September 2015. All patients were treated with RT (<54 Gy or ≥54 Gy) after an initial two cycles of P-GEMOX, and following two consolidation cycles. The primary endpoints were objective response rate and complete remission rate (CR). The secondary endpoints were 2-year overall survival (OS), 2-year progression-free survival (PFS), and toxicity.
Results: Thirty-three patients completed total therapy, which resulted in 94.3% of response rate that included 28 patients (80.0%) with CR and five patients (14.3%) with partial response (PR). Two (5.7%) patients progressed during therapy and six (17.1%) progressed during follow-up. The 2-year OS was 82.9%, and the 2-year PFS was 77.1%. Patients with CR, low circulating EBV DNA load (≤6.1 × 107 copies/ml), low NKIPI (score 0–1), and high RT dose (≥54 Gy) were independent predictors of better prognosis. Grade 3 toxicities were few; only four (11.4%) patients experienced grade 4 toxicities. No treatment-related deaths were observed.
Conclusions: The research showed that the treatment of P-GEMOX combined with RT was a tolerable and effective treatment for localized nasal natural killer/T-cell lymphoma.
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL), nasal-type, is a distinct entity according to World Health Organization classification of tumors of the hematopoietic and lymphoid tissues. ENKTL is a highly invasive tumor with a short doubling time and poor prognosis. Quantification of circulating EBV DNA is an accurate biomarker of tumor load. This disease is relatively rare in the U.S.A. and Europe, where it accounts for less than 1% of all non-Hodgkin’s Lymphoma (NHL) cases. It is more common in Asia and South America, where it accounts for 3–8% of all NHL cases [Citation1,Citation2]. Owing to its rarity, it is difficult to do a randomized, controlled trial. The mechanism of lymphomagenesis and the optimal first-line treatment for these tumors have not yet established.
The main treatment for late stage ENKTL is chemotherapy. Chemotherapy based on the CHOP regimen is used most frequently, but the response rate is low, and the 5-year overall survival (OS) is only 7–25% [Citation3]. Thus, more and more studies keep focusing on localized ENKTL (stage I–II) because of its potential curability. Most localized ENKTL patients present as an early stage disease in the upper aerodigestive tract from the nasal cavity to the hypopharynx [Citation4,Citation5]. Radiotherapy (RT) has been considered the preferred first-line therapy for early stage ENKTL because of its high response rate [Citation6]. But, these early stage patients treated with RT alone are still at high risk for local relapse and systemic failure, its reported 5-year OS has been shown to range from 40.1 to 66% [Citation6–8]. Therefore, the combination of RT and chemotherapy can be considered an effective treatment option for localized ENKTL to reduce the risk of recurrence, and the promising results of recent prospective studies with concurrent chemoradiotherapy support this treatment strategy [Citation9,Citation10]. However, treatment outcomes with conventional chemotherapy regimen (CHOP or CHOP-like: cyclophosphamide, doxorubicin, vincristine, and prednisone) followed by RT for early stage ENKTL are dismal: reported 5-year OS is less than 50% [Citation7,Citation11,Citation12]. Thus, nonanthracycline-based chemotherapy regimens have been explored to get better survival benefit. In particular, l-asparaginase-based regimens showed outstanding response rates in patients with both refractory/relapsed and localized ENKTL [Citation13–15]. But l-asparaginase treatment has two main disadvantages: the need for frequent intramuscular injection, and a very high rate of allergic reactions, which have become the key limiting factors of its use [Citation16]. Pegaspargase (PEG-Asp) is a polyethylene glycol-modified version of l-asparaginase with reduced immunogenicity, longer plasma half-life and more operational convenience [Citation17]. However, there are quite few reports on the application of PEG-Asp-based regimens to the treatment of early stage ENKTL. Thus, we conducted a retrospective study of combined first-line P-GEMOX (Pegaspargase, oxaliplatin, and gemcitabine) and RT for newly diagnosed, localized ENKTL to examine the efficacy and tolerance of this regimen.
Materials and methods
The subjects were ENKTL patients who received P-GEMOX as first-line treatment from October 2011 to September 2015 in our center. We retrospectively reviewed their medical records. All patients had a pathological and clinical diagnosis of ENKTL.
Eligibility criteria
Eligible criteria were as follows: Ann Arbor stage IE or IIE; with primary site in the upper aerodigestive tract; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; have adequate hematologic function (i.e. hemoglobin > 9.0 g/l, absolute neutrophil count > 1500/μl, and platelets > 100 000/μl), hepatic function (i.e. total bilirubin two times the upper limit of normal, alanine aminotransferase and aspartate aminotransferase levels 2.5 times the upper limit of normal), renal function (i.e. serum creatinine 1.5 mg/dl, creatinine clearance 50 ml/minute), and normal electrocardiogram results. Excluding criteria were pregnancy or lactation, any complication to prevent full compliance with the study, and patients with ENKTL not located in upper aerodigestive tract sites, such as in skin or the gastrointestinal tract.
Treatment protocol
All diagnosed patients were treated with P-GEMOX regimen. The regimen was repeated every 3 weeks and administrated as follows: pegaspargase, 2500 IU/m2 intramuscularly with no skin test, on day 1; gemcitabine, 1000 mg/m2 intravenously, on days 1 and 8; oxaliplatin, 130 mg/m2 intravenously, on day 1. After two cycles of chemotherapy, RT was started, and then chemotherapy was given for another two cycles at 1 week after the completion of RT, resulting in a maximum total of four cycles of P-GEMOX. All drugs were administered only if the absolute neutrophil count was >1500/μl, the platelet count was >75 000/μl, and liver dysfunction < Grade 2, before each cycle. If patients developed grade 4 hematologic toxicity, then the doses of all chemotherapy drugs were reduced by 20% in all subsequent cycles.
Primary IFRT was delivered using 6-MeV linear accelerator using 3-dimensional conformal treatment planning. The IFRT dose was 48–56 grays (Gy), with 2 Gy a day and five fractions each week. The gross tumor volume (GTV) was determined according to CT or MRI analysis and endoscopic findings. The clinical target volume (CTV) consisted of the GTV and adjacent structures with potential involvement: CTV of limited stage IE disease included the nasal cavity, bilateral frontal ethmoid sinuses, and the ipsilateral maxillary sinus. For patients with extensive stage IE disease, CTV was extended to the involved organs and/or tissues. For stage IIE disease, CTV included the affected cervical lymph node area. The planning target volume was generated by adding a 5 mm margin to the CTV. RT was postponed until the toxicity was reduced to grade 2 if one or more of the following adverse events were observed: grade 4 leukopenia or neutropenia, platelet count <25 000/μl, any grade 3 nonhematologic toxicities except for mucositis or dysphagia related to radiation, and/or ECOG performance status ≥3.
Evaluation and response
Baseline evaluation included a complete history, physical examination, complete blood count, serum biochemistry with lactate dehydrogenase (LDH), serum EBV DNA copies, bone marrow puncture and biopsy, nasopharyngeal endoscopic examination of the nasal and oral cavities, magnetic resonance imaging with contrast of the head and neck, and computed tomography with contrast of the thorax, abdomen, and pelvis, PET–CT was used when it was difficult to give an accurate stage and after completion of the whole treatment. The cases were staged according to the Cotswold modification of the Ann Arbor staging system.
All hematological tests were repeated before every chemotherapy cycle. Imaging examinations were repeated before RT, after RT, at the end of treatment, and every 3 months thereafter to monitor disease progression.
We calculated the NK/T-cell lymphoma prognostic index (NKIPI), which includes the presence of ‘B’ symptoms, lesions at stage III or IV, elevated serum LDH concentration, and lymph node involvement.
Treatment response was assessed according to the standardized response criteria for NHL. Complete remission rate (CR) was defined as no evidence of disease, bone marrow and serum EBV DNA negative which had been confirmed with PET–CT. Partial response (PR) was defined as ≥50% decrease in sum of the product of the diameters (SPDs) of masses and no new lesions. Stable disease (SD) was defined as a patient who failed to attain CR or PR but did not fulfill those criteria for progressive disease (PD). PD was defined as appearance of new sites or ≥50% increase in SPD of previous lesions from nadir.
The primary endpoints were CR and objective response rate (ORR), which was calculated as the proportion of patients classified as having CR or PR. The secondary endpoints were 2-year OS and 2-year progression-free survival (PFS), and toxicity, which were evaluated according to the National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0.
Statistical analysis
Retrospective studies have shown that the CR rate for CHOP-like chemotherapy with RT for newly diagnosed, localized nasal I/II ENKTL was approximately 50% [Citation3,Citation7,Citation11,Citation18]. Our study was designed with a hypothesis that the combined treatment P-GEMOX and RT is superior to the historical control. Our designed combined treatment P-GEMOX and RT was expected to have a CR rate of approximately 80%, and thus would be more effective than the historical control.
OS was measured from the date of the treatment started until the date of death or the last follow-up. PFS was measured from the date of the treatment started until the date of disease progression or death from any cause.
Survival estimates were calculated using the Kaplan–Meier method. In subgroup analyses, survival curves were compared by the log-rank test. A two-sided p value of <0.05 was considered significant. All analyses were performed using SPSS v.17.0 (SPSS Inc., Chicago, IL, U.S.A.)
Result
Patient characteristics
During the period from August, October 2011 to September 2015 in our hospital, there were 43 patients who were diagnosed as ENKTL. Among these, eight patients were excluded, the reasons were: five patients were late stage (stage III or IV), one with chronic active hepatitis B and abnormal liver function (aminotransferase levels five times the upper limit of normal), one with uremia and one with severe heart failure and uncontrolled diabetes. Thirty-five (81.4%) patients met the eligibility criteria and were evaluated in this study, and 33 patients completed treatment according to the schedule. The characteristics of the patients are summarized in .
Table 1. Patient demographic and clinical characteristics.
Treatment response and survival
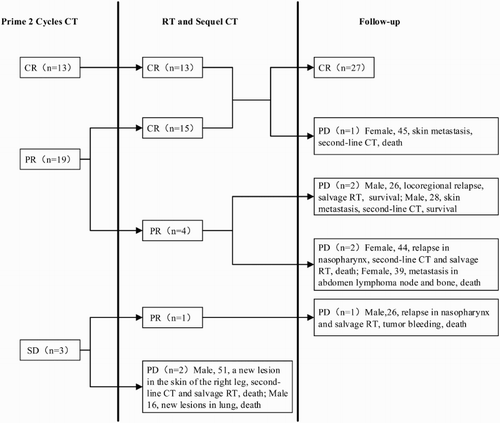
The treatment outcome was summarized in . A total of 136 cycles of P-GEMOX regimen were administrated. Thirty-three patients completed the RT expect two patients developed disease progression. After the first two cycles of chemotherapy, there were 13 CRs (37.1%), 19 PRs (54.3%), and three patients had stable disease (8.6%); no patient experienced disease progression during the first two cycles of chemotherapy. After RT and subsequent chemotherapy, 13 patients remained in CR, 15 patients with PR achieved CR, four patients remained in PR, and one patient with SD achieved PR. Two patients progressed during RT process. At the end of treatment, the ORR was 94.3%, including 28 patients with CR (80.0%) and five with PR (14.3%). For two patients (5.7%), the disease progressed during therapy.
Relapse and survival
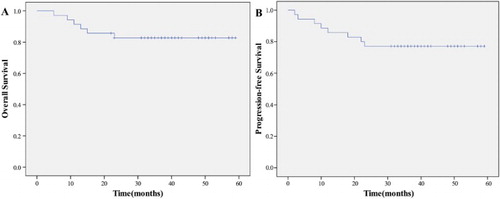
The median follow-up time for the 35 patients was 36 months (range, 5–59 months). During the therapy and follow-up period, eight of 35 patients (22.9%) experienced disease progression. One patient who was in complete remission at the end of treatment had new lesions in whole body skin 12 months after the end of treatment, and she was treated with second-line regimen and died 5 months later. Five patients who were still in partial remission at the end of treatment, experienced disease progression in the follow-up period: the first patient developed locoregional relapse, accepted salvage RT and still survives. The second patient had lesions in skin, accepted second-line chemotherapy and still survives. The third patient experienced locoregional relapse in the left maxillary sinus outside the border of the radiation field 4 months after the end of treatment; he was treated with the second-line chemotherapy regimen and salvage RT but experienced systemic progress and died 7 months later. The fourth patient developed metastasis in abdomen lymphoma node and bone 5 months after the end of treatment and died 1 month later. The fifth patient developed locoregional relapse in nasopharynx and died 4 month later because of tumor bleeding during salvage RT. Two patients progressed during therapy: one had new lesions in the lungs 2 month later and died at 5 months of follow-up, the other patient had a new lesion in the skin of the right leg 3 months later; she was treated with salvage regimen and RT but developed systematic progression finally and died at 9 months of follow-up. All other patients are being followed. At the time of this report, the 2-year OS was 82.9%, the 2-year PFS was 77.1%, and median OS has not been reached ().
Prognosis
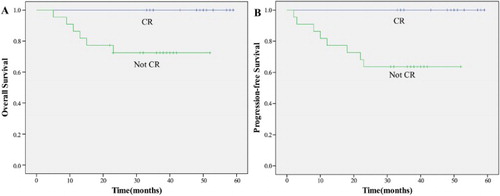
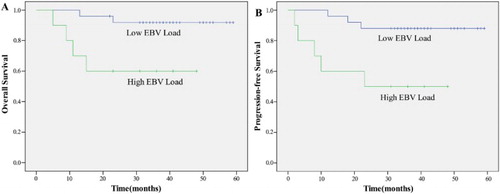
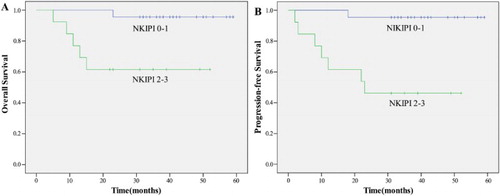
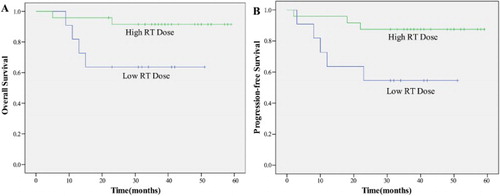
A univariate analysis revealed that patients with CR, low circulating EBV DNA load, NKIPI score 0–1, and RT dose ≥54 Gy were significant predictors of both OS and PFS (p < 0.05) (–).
Toxicity
The adverse effects of P-GEMOX are summarized in . Grade 1 and 2 toxicities were frequent during P-GEMOX treatment. The major side effects were leukopenia, anemia, thrombocytopenia, nausea, vomiting, mucositis, liver dysfunction, and hyperbilirubinemia. Grade 3 and 4 toxicities were few. Eleven patients (31.4%) experienced grade 3 leukopenia and five patients (14.3%) experienced grade 3 thrombocytopenia, two patients (5.7%) experienced grade 4 leukopenia. Grade 1 and 2 mucositis and dermatitis were frequent during IFRT, nine patients (25.7%) experienced grade 3 mucositis and two patients (5.7%) experienced grade 4 mucositis. There were no treatment-related deaths. Two of 35 patients (5.7%) had a delay in chemotherapy because of an abnormal grade 3 liver dysfunction and grade 4 thrombocytopenia, respectively. No patients suffered from the following problems: pancreatitis, elevated glucose level, or blood clotting disorder because of receiving pegaspargase.
Table 2. Toxicity with the combined treatment.
Discussion
Recent years, the combination of RT and chemotherapy has been recommended as an effective treatment option for localized ENKTL [Citation18,Citation19]. But for a long time, there remains a lack of consensus on selection of chemotherapy regimen because of the primary drug resistance of NK/T lymphoma cells. The mechanism may be related to the high expression of the multidrug-resistant P-glycoprotein in lymphoma cells, which results in drug efflux and treatment resistance [Citation20]. In previous studies, anthracycline-based chemotherapy like CHOP followed by RT resulted in a CR rate of approximately 50% [Citation11,Citation18]. Thus, CHOP and CHOP-like regimen are not effective for ENKTL and have been abandoned. Under these circumstances, oncologists put great effort into exploring more effective nonanthracycline-based regimens with drugs not affected by P-glycoprotein. For example, the frontline use of IMEP (ifosfamide, methotrexate, etoposide, and prednisolone), DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) and VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone) produced 77–80% CR and 81–83% ORR in newly diagnosed stage I/II ENKTL [Citation10,Citation21–23]. However, hematologic toxicity of greater than grade 3 was frequently observed with those intensified regimens [Citation10,Citation22,Citation23]. Thus, further investigation is required to explore less toxic regimens that retain efficacy.
l-asparaginase (l-Asp) is one of those new explored drugs with a special anticancer mechanism. It can hydrolyze and exhaust serum asparagines in NK/T-cell lymphoma cells, which cannot synthesize l-Asp by themselves, and finally produce an anticancer effect [Citation24]. Previous studies have identified that l-Asp-containing regimens (such as SMILE and AspaMetDex) showed outstanding response rates in ENKTL [Citation25,Citation26]. Although high response rate can be attainted with these regimens, the toxicities are nearly unacceptable [Citation26].
Two recent studies with promising results also provided a powerful support to the efficacy of l-Asp-containing regimens in localized ENKTL. The first study conducted a very similar regimen GELOX (l-asparaginase, gemcitabine, and oxaliplatin) with RT in early stage ENKTL and produced an ORR of 96.3%, which included an CR rate of 74.1% and a PR rate of 22.2%, the 2-year OS and the 2-year PFS are both 86% [Citation14,Citation27]. The second is the frontline use of LVP (l-asparaginase, vincristine, and prednisone) with RT, which also is a nonanthracycline-based chemotherapy regimen, produced a response rate of 88.5%, which included an 80.8% CR rate and a 7.7% PR rate, the 2-year OS is 88.5%, and the 2-year PFS is 80.6% [Citation13].
Recent years, a polyethylene glycol-modified version of l-Asp, pegaspargase (PEG-Asp), with reduced immunogenicity and more convenience has been explored [Citation17,Citation28]. It has a plasma half-life of about 5.5 days, substantially longer than that of l-Asp (approximately 20 hours). PEG-Asp is advantageous as it does not require a skin test, needs far fewer drug administrations, and can be delivered more conveniently. Previous studies showed that PEG-Asp appears to be safe and effective in patients with acute lymphoblastic leukemia [Citation29]. But there are few relevant reports about PEG-Asp-based regimens with RT applied to untreated, localized ENKTL.
Gemcitabine is a pyrimidine antimetabolite with activity against solid tumors and pretreated T-cell lymphoma patients [Citation30]. Combined with other antitumor agents like oxaliplatin, gemcitabine has also shown substantial clinical activity in aggressive B cell and T cell lymphoma [Citation31,Citation32]. However, therapeutic strategies, containing gemcitabine in the treatment of ENKTL, were scarcely explored and identified.
In our study, we combined PEG-Asp, gemcitabine, and oxaliplatin to form a new regimen: P-GEMOX. Regimen GEMOX (gemcitabine and oxaliplatin) has been proved safe and effective to refractory or relapse B-cell lymphoma patients and is wildly used in the second-line treatment of NHL [Citation33]. Our regimen takes advantage of the combination of gemcitabine, oxaliplatin, and pegaspargase, all of which are nonanthracycline drugs that demonstrated significant activity and nonoverlapping toxicity in previous studies. The purpose of this retrospective study is to demonstrate the safety and effectiveness of our new chemotherapeutic regimen (P-GEMOX) applied in localized ENKTL with concurrent RT.
In our study, we achieved an encouraging response rate: the ORR was 94.3% (33 of 35 patients) and the CR rate was 80.0%, the 2-year OS was 82.9%, and the 2-year PFS was 77.1%, which is significantly higher than conventional regimen, indicating that P-GEMOX chemotherapy was more active than anthracycline-based chemotherapy, and median OS has not been reached. Moreover, this result is comparable to those from current reports described above. Besides, our regimen is safer and more convenient, because it did not need frequent intramuscular injection, and had almost no allergic reactions.
P-GEMOX showed remarkable antitumor activity for untreated stages I/II ENKTL in our study. It is noteworthy that almost all (33 of 35, 94.3%) patients have an amazing rapid response to P-GEMOX regimen after the first two cycles of chemotherapy. Thus, the planned RT was delivered on time and almost all patients (33 of 35, 94.3%) complete the entire course of treatment. Patients who attained a complete response had significantly longer PFS and OS. Although the follow-up in our study was relatively short, the recurrence peak did not appear 2 years after the completion of treatment.
The dose of RT keeps controversial. The majority of previous studies used a radiation dose of 50 Gy, because it was reported that a radiation dose of 45 Gy was significantly associated with local relapse [Citation12,Citation34]. However, there is no consensus on the minimum required dose, although some studies reported that at least 52 or 54 Gy might be required to obtain in-field control in patients with localized ENKTL [Citation35,Citation36]. Our study used a dose of 48–56 Gy to ensure the effectiveness and achieved an 80% (28 of 35 patients) CR rate. There is a very low rate (three of 35, 8.6%) of local relapse occurred in the radiation field during the follow-up period. Through Kaplan–Meier analysis, RT dose were significant predictors of both OS and PFS (p < 0.05), high RT dose (≥54 Gy) were independent predictors of better prognosis.
A recent study found that RT dose could be safely reduced with the same decent survival outcomes and decreased RT side effects for patients who received CR after induction chemotherapy [Citation37]. In our study, 13 patients achieved CR after first two cycles of P-GEMOX regimen. Among these patients, two of them were treated with lower dose RT and 11 of them were treated with higher dose RT. All 13 patients survived more than 2 years. Twenty-two patients achieved PR or SD, nine of them were treated with lower dose RT and 13 were treated with higher dose RT. Log-rank test showed that among those who cannot achieve CR, the patients with higher dose could have better outcome, but there is no statistic difference (p = 0.11). Although the sample size of our study need to be expanded to give a more convincing result, it reminds us that: whether the patients with CR after induction chemotherapy could benefit from lower RT dose and the patients without CR should be treated with higher RT dose? We will include more patients in our study to validate this point in the future.
Besides, P-GEMOX chemotherapy regimen, combined with RT, was well tolerated in our study. Thirty-three patients (94.3%) completed the planned treatment. Grade 3/4 toxicity, mainly developed in leukocytopenia and mucositis related to radiation, could be treated with granulocyte colony-stimulating factor and parenteral nutrition. No severe toxicity was observed, such as allergic reactions, pancreatitis, or thrombosis. No treatment-related death was reported.
In our study, we observed that the clinical course and treatment outcome can be different even within the same group of patients with localized disease because each tumor can have different aggressive features. Thus, accurate assessment of poor prognostic factors is important for discriminating patients at high or low risk of relapse. The International Prognostic Index (IPI) is not an ideal assessment instrument because localized ENKTL mainly occurs in middle-aged men with good performance status, thus the majority of patients have low or low-intermediate risk [Citation38,Citation39]. NKIPI should be more accurate to distinguish patient, for patients with poor prognostic factors, such as a high NKIPI, autologous stem cell transplantation (ASCT) during remission and additional treatments with central nervous system prophylaxis may be beneficial. Therefore, initial experience suggested that patients with poor prognostic factors should be considered for ASCT at the time of first CR. In large-scale reports from Japan and Korea, the study showed that the advantage of ASCT was significant only for patients with a high-risk prognostic index [Citation40]. Our study showed high NKIPI (score 2–3) was an independent predictor of poor prognosis (p < 0.05). A recent study shows that post-treatment Deauville score on PET–CT scan and the presence of Epstein–Barr virus DNA can predict the risk of treatment failure in patients with ENKTL [Citation41]. The authors stratified patients into three groups based on risk of treatment failure: a low-risk group (post-treatment Epstein–Barr virus negativity and post-treatment Deauville score of 1–2), a high-risk group (post-treatment EBV negativity with a Deauville score of 3–4, or post-treatment Epstein–Barr virus positivity with a Deauville score of 1–2), and treatment failure (Deauville score of 5 or post-treatment Epstein–Barr positivity with a Deauville of score 3–4). Regrettably, our study did not test post-treatment EBV level, but we reviewed our patient’s post-treatment PET–CT scan. The number of patients with different score and the associated treatment failure proportion were: 10 patients had a Deauville score of 1 and with 0 (0%) failure, 16 had score of 2 with 1 (6.25%) failure, five had score of 3 with 2 (40%) failure, two had score of 5 with 2 (100%) failure. These results were similar to that of the above study. We will continue to evaluate this new classification in our next validation study. Another recent study established a new prognostic model (PINK and PINK-E) based on nonanthracycline chemotherapy to predict outcomes in ENKTL [Citation42]. Because all the patients included in our study were stage I–II, nasal-type, without distant lymph-node involvement and most of them were younger than 60 years, when the PINK model was applied to our group: 31 were stratified to low-risk level and 26 of them survived over 2 years (83.8%), four were stratified to intermediate-risk level and all of them survived over 2 years (100%). When PINK-E model was applied to our group: 33 were stratified to low-risk level and 27 of them survived over 2 years (81.8%), two were stratified to intermediate-risk level and all of them survived over 2 years (100%). There were no patients stratified to high-risk level. These results were consistent with the above study.
Besides, some previous studies suggested that plasma EBV DNA load at presentation is associated with clinical disease stage and treatment response, and patients with a high EBV DNA load (≥6.1 × 107 copies/ml) have a poorer prognosis [Citation43]. Our result confirmed this point: EBV DNA load ≥6.1 × 107 copies/ml were significant predictors of poor outcome in both OS and PFS (p < 0.05).
Our results suggested that PEG-Asp-based chemotherapy presented an acceptable tolerance and a potential short-term outcome in patients with early stage nasal-type ENKTL. But, the result of our study must be viewed cautiously owing to its limitations. Owing to its rarity and to these unique characteristics, most data concerning ENKTL are from small case series or retrospective analyses, the precise role of treatment strategy, combining RT and chemotherapy, should be further optimized in large-scale randomized controlled trials in future based on the promising results of recent studies with concurrent chemoradiotherapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Wen Wei is 31 years old. She has obtained her MD. She is a medical oncologist at the Department of Chemotherapy, Sichuan Cancer Hospital, Chengdu, China. Her research field is fundamental and clinical study in lymphoma.
Ping Wu is 48 years old. She has obtained her MD. She is a medical oncologist at the Department of Chemotherapy, Sichuan Cancer Hospital, Key Laboratory of Cancer Prevention and Therapy of Sichuan, China. She is a clinical trial expert and a statistical expert in cancer.
Li Li is 45 years old. She has obtained her MD. She is a hematologist at the Department of Chemotherapy, Sichuan Cancer Hospital, Chengdu, China. Her research field is fundamental study and PBSCT in malignant disease of hematology.
Zhi-hui Zhang is 55 years old. He has obtained his MD and PhD. He is a medical oncologist at the Department of Medical Oncologist, Sichuan Cancer Hospital, Chengdu, China. His research field is fundamental research in lymphoma.
References
- Liu J, Song B, Fan T, et al. Pathological and clinical characteristics of 1,248 non-Hodgkin’s lymphomas from a regional cancer hospital in Shandong, China. Asian Pac J Cancer Prev. 2011;12(11):3055–3061.
- Chakrabarti S, Sarkar S, Goswami BK, et al. Hodgkin’s and non-Hodgkin’s lymphomas in an indian rural medical institution: comparative clinicopathologic analysis. Asian Pac J Cancer Prev. 2010;11(6):1605–1608.
- Li CC, Tien HF, Tang JL, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer. 2004;100(2):366–375. doi: 10.1002/cncr.11908
- Tse E, Kwong Y-L. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997–5005. doi: 10.1182/blood-2013-01-453233
- Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206–214. doi: 10.1093/annonc/mdi037
- Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000;18(1):54–63.
- Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998;16(1):70–77.
- Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(1):181–189. doi: 10.1200/JCO.2005.03.2573
- Aviles A, Neri N, Fernandez R, et al. Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma: results of a clinical trial. Med Oncol. 2013;30(3):637. doi:10.1007/s12032-013-0637-1
- Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27(35):6027–6032. doi: 10.1200/JCO.2009.23.8592
- Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001;12(3):349–352. doi: 10.1023/A:1011144911781
- Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27(33):5594–5600. doi: 10.1200/JCO.2009.23.8295
- Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of ‘sandwich’ L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118(13):3294–3301. doi: 10.1002/cncr.26629
- Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119(2):348–355. doi: 10.1002/cncr.27752
- Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. doi: 10.1182/blood-2012-05-431460
- Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18(7):1525–1532.
- Alfieri DR. Pegaspargase. Pediatr Nurs. 1995;21(5):471–490.
- Wu X, Li P, Zhao J, et al. A clinical study of 115 patients with extranodal natural killer/T-cell lymphoma, nasal type. Clin Oncol (R Coll Radiol). 2008;20(8):619–625. doi: 10.1016/j.clon.2008.05.011
- Guo Y, Lu JJ, Ma X, et al. Combined chemoradiation for the management of nasal natural killer (NK)/T-cell lymphoma: elucidating the significance of systemic chemotherapy. Oral Oncol. 2008;44(1):23–30. doi: 10.1016/j.oraloncology.2006.11.020
- Wang B, Li XQ, Ma X, et al. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83(10):795–799. doi: 10.1002/ajh.21256
- Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol. 2015;94(3):437–444. doi: 10.1007/s00277-014-2228-4
- Nomura E, Isoda K, Yamanaka K, et al. Extra nodal NK/T-cell lymphoma nasal type that responded to DeVIC combination chemotherapy. J Dermatol. 2005;32(3):204–209. doi: 10.1111/j.1346-8138.2005.tb00746.x
- Zhou Z, Li X, Chen C, et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. 2014;93(11):1889–1894. doi: 10.1007/s00277-014-2136-7
- Ando M, Sugimoto K, Kitoh T, et al. Selective apoptosis of natural killer-cell tumours by l-asparaginase. Br J Haematol. 2005;130(6):860–868. doi: 10.1111/j.1365-2141.2005.05694.x
- Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. doi: 10.1182/blood-2010-09-307454
- Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410–4416. doi: 10.1200/JCO.2011.35.6287
- Wang L, Wang WD, Xia ZJ, et al. Combination of gemcitabine, L-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up. Med Oncol. 2014;31(3):860. doi:10.1007/s12032-014-0860-4
- Dinndorf PA, Gootenberg J, Cohen MH, et al. FDA drug approval summary: pegaspargase (oncaspar(R)) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist. 2007;12(8):991–998. doi: 10.1634/theoncologist.12-8-991
- Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007;109(7):2744–2750.
- Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2010;21(4):860–863. doi: 10.1093/annonc/mdp508
- Evens AM, Rosen ST, Helenowski I, et al. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol. 2013;163(1):55–61. doi: 10.1111/bjh.12488
- Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica. 2013;98(11):1726–1731. doi: 10.3324/haematol.2013.090597
- El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18(8):1363–1368. doi: 10.1093/annonc/mdm133
- Tham IW, Lee KM, Yap SP, et al. Outcome of patients with nasal natural killer (NK)/T-cell lymphoma treated with radiotherapy, with or without chemotherapy. Head Neck. 2006;28(2):126–134. doi: 10.1002/hed.20314
- Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70(1):166–174. doi: 10.1016/j.ijrobp.2007.05.073
- Koom WS, Chung EJ, Yang WI, et al. Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys. 2004;59(4):1127–1137. doi: 10.1016/j.ijrobp.2003.12.006
- Wang L, Bi XW, Xia ZJ, et al. Radiation dose reduction for patients with extranodal NK/T-cell lymphoma with complete response after initial induction chemotherapy. OncoTargets Ther. 2016;9:5875–5881. doi: 10.2147/OTT.S116591
- Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–618. doi: 10.1200/JCO.2005.04.1384
- Kim TM, Park YH, Lee S-Y, et al. Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage IE/IIE extranodal NK/T-cell lymphoma, nasal type. Blood. 2005;106(12):3785–3790. doi: 10.1182/blood-2005-05-2056
- Kim HJ, Bang SM, Lee J, et al. High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant. 2006;37(9):819–824. doi: 10.1038/sj.bmt.1705349
- Kim SJ, Choi JY, Hyun SH, et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol. 2015;2(2):e66–e74. doi: 10.1016/S2352-3026(15)00002-2
- Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389–400. doi: 10.1016/S1470-2045(15)00533-1
- Suzuki R, Yamaguchi M, Izutsu K, et al. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood. 2011;118(23):6018–6022. doi: 10.1182/blood-2011-05-354142