ABSTRACT
Objectives: CD25 has been reported to be highly expressed in leukemia stem cells and correlated with adverse outcomes in young patients with acute myeloid leukemia (AML). However, the significance of CD25 expression in elderly patients with AML has not yet been investigated.
Methods: We retrospectively analyzed 154 newly diagnosed AML patients aged 60 years or over by flow cytometry.
Results: CD25-positive AML was characterized by high white blood cell counts, secondary AML, rare favorable karyotypes, and positivity for CD34 and CD7 antigens, compared with CD25-negative AML. CD25 positivity was significantly correlated with an inferior complete remission (CR), event-free survival (EFS), and overall survival. Multivariate analysis showed CD25 positivity to be a significant prognostic predictor of CR and EFS. A regimen of low-dose cytarabine and aclarubicin combined with granulocyte-colony-stimulating factor (CAG) led to higher CR rates in the CD25-positive AML patients than intensive chemotherapies. CD25 expression was increased at relapse and in the development of leukemic status from myelodysplastic syndrome or myeloproliferative neoplasm.
Discussion: An effective treatment strategy for elderly patients with CD25-positive AML has not been established. Further studies are needed to evaluate the effect of a CAG regimen and allogenic stem cell transplantation in patients.
Conclusion: CD25 is an independent prognostic factor in elderly AML patients. Alternative therapies for CD25-positive elderly AML patients are needed.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of elderly patients and approximately half of all adult patients are aged 65 years or more at diagnosis [Citation1]. Elderly patients with AML, generally defined as those older than 60 years, have distinct features that are biologically and clinically distinct from young patients with AML [Citation2–4]. In elderly patients, the higher frequency of unfavorable cytogenetics, secondary AML, and overexpression of the multidrug resistance 1 gene are associated with a lower response to chemotherapy. Moreover, poor general condition and significant comorbidity contribute to an increase in complications and less tolerance to treatment, leading to poor clinical outcomes. Elderly patients with AML are heterogeneous; some show long-term survival after intensive chemotherapy, while others do not. Therefore, it is important to determine a new prognostic marker for therapeutic decision-making in elderly patients with AML.
CD25, the alpha chain of the interleukin 2 receptor, is normally expressed on activated T-lymphocytes and is involved in the growth and differentiation of T-lymphocytes [Citation5]. Elevated CD25 expression has been observed in a variety of cancers, including leukemia, lymphoma, colon, ovarian, prostate, and melanoma, which are all associated with a poor prognosis for patients [Citation6]. To date, it has been reported that CD25 is highly expressed in leukemia stem cells (LSCs) [Citation7], and CD25-positive AML blasts have a LSC-like gene expression profile [Citation8]. In young AML patients, CD25 expression is an independent adverse prognostic factor for response and survival rate and is positively correlated with FMS-like tyrosine kinase-3-internal tandem duplication (FLT3-ITD) mutation [Citation8–11].
However, the significance of CD25 expression in elderly patients with AML has not yet been investigated. In this study, we retrospectively evaluated CD25 expression and its prognostic efficacy in untreated elderly patients with AML.
Materials and methods
Patients
From January 2005 to December 2013, we evaluated 154 consecutive patients with AML aged 60 years or more, who were newly diagnosed at Jichi Medical University hospital. Clinical data were reviewed retrospectively and immunophenotyping was performed on fresh samples at diagnosis. Cytogenetic analysis was performed using standard methods of G-banding and cytogenetic abnormalities were grouped according to the Medical Research Council [Citation12]. The diagnosis of AML was based on morphology, immunophenotyping, and cytogenetics. Diagnosis of de novo AML was made according to the French–American–British (FAB) classification system. Normal bone marrow blasts were obtained from six healthy volunteers. The study protocol was approved by the Bioethics Committee of Jichi Medical University.
Treatment
Patients diagnosed with acute promyelocytic leukemia (APL) were initially treated with all-trans retinoic acid (ATRA) combined with anthracycline-based chemotherapy (13 patients). After physicians assessments based on the comorbidity and performance status (PS), initial induction treatments for non-APL AML patients were performed as follows: daunorubicin and cytarabine (61 patients), idarubicin and cytrabine (2 patients), CAG regimen (low-dose cytarabine, aclarubicin, and granulocyte-colony-stimulating factor (G-CSF)) (38 patients) [Citation13], azacitizine (2 patients), cytarabine (1 patient), hydroxyurea (1 patient), and supportive care (36 patients). Allogenic stem cell transplantation (SCT) was used at relapse (1 patient) and induction failure (5 patients).
Responses were evaluated according to the recommendations of the International Working Group criteria [Citation14]. If patients did not achieve complete remission (CR) after the first course of induction chemotherapy, a second course of induction chemotherapy was performed after approximately a 3- to 4-week interval. If patients did not achieve CR after two courses of chemotherapy, they were determined to have induction failure.
Flow cytometry
Mononuclear cells were separated from an aliquot of the bone marrow samples and used for flow cytometry (FCM) with a CD45 gate. Cells were stained with a fluorescein isothiocyanate-conjugated monoclonal antibody and/or a phycoerythrin-conjugated monoclonal antibody, and peridinin chlorophyll protein-conjugated CD45. Monoclonal antibodies used in this study were as follows: CD25 (Becton Dickinson Biosciences, San Jose, CA), CD7 (Becton), CD11b (Beckman Coulter, Hialeah, FL), CD13 (Beckman), CD14 (Beckman), CD15 (Becton), CD33 (Beckman), CD34 (Becton), CD36 (Beckman), CD45 (Becton), CD56 (Becton), and HLA-DR (Beckman). For negative controls, cells were stained with isotype-matched control antibodies. FCM was performed to analyze blast phenotypes in the blast region. Phenotypes of cells were analyzed using a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA).
Statistical analysis
The chi-square test was used to compare the two groups for categorical data and the Student’s t-test for continuous data. Event-free survival (EFS) was calculated using the date of diagnosis until induction failure, relapse, or death from any cause. Overall survival (OS) was calculated using the date of diagnosis until death from any cause. Patients still alive or lost to follow-up were censored at the date they were last known to be alive. EFS and OS were estimated using the Kaplan–Meier method, and were compared using the log-rank test. Multivariate analysis was performed using logistic regression analysis and the Cox proportional hazard regression analysis. p-values were two-sided and regarded as significant if they were less than 0.05. Data were analyzed using StatMate software version IV for Windows (ATMS, Tokyo, Japan).
Results
CD25 expression in elderly AML patients
The median CD25 expression on AML blasts was 0.7% (range: 0–75.4%) and most patients did not express CD25 at diagnosis ((a)). CD25 expression on bone marrow blasts from healthy volunteers did not exceed 10% (median: 3.2%, range: 1.5–5.6%), we adapted the cut-off value of 10%, and AML with CD25 expression more than 10% was defined as CD25-positive AML (CD25+ AML) ((b)).
Figure 1. CD25 expression in healthy volunteers and patients with AML (A), in a patient with CD25-positive AML (B), in patients with AML at diagnosis and relapse (C), and in patients with secondary AML and myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN) (D). Leukemic cells from an AML patient, which were identified by forward and side scatter properties, expressing CD25 and coexpressing CD34 and CD7 at significant levels (B).
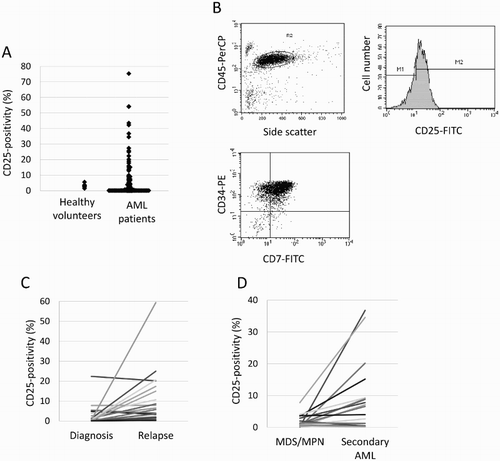
In this study, 69 patients achieved CR, but 39 of them (56.5%) relapsed after this. CD25 expression was higher at relapse than at diagnosis (n = 25, median: 3.6 vs. 0.6%, p = 0.027), and AML blasts of six patients at relapse showed CD25 expression ((c)).
As shown in (d), CD25 expression increased after disease progression from myelodysplastic syndrome (MDS) or myeloproliferative neoplasm to secondary AML (n = 17, median: 1 vs. 7.1%, p = 0.01). CD25 expression in patients with secondary AML (n = 30) was higher than that in patients with de novo AML (n = 124) (median: 4.4 vs. 0.6%, p = 0.044).
Clinical characteristics of CD25+ elderly AML patients
In this cohort, the median age was 69 years (range, 60–85 years). Of the 154 patients with AML, CD25 positivity was confirmed in 21 patients (13.6%). Patient characteristics are summarized in . There was no statistical difference in sex, age, and marrow blasts at diagnosis in the two groups. Compared with CD25-negative AML patients (CD25− AML), CD25+ AML patients were characterized by poor PS (p = 0.003), high white blood cell (WBC) count (p = 0.078), a history of hematological disorders (p = 0.08), and a lack of favorable cytogenetics (p = 0.08). Patients with CD25+ AML showed a high frequency of the FAB M0 subtype (19 vs. 7%, p = 0.14), a low frequency of the FAB M2 subtype (10 vs. 32%, p = 0.06), and no cases of the FAB M3 subtype (0 vs. 10%, p = 0.28).
Table 1. Patient characteristics.
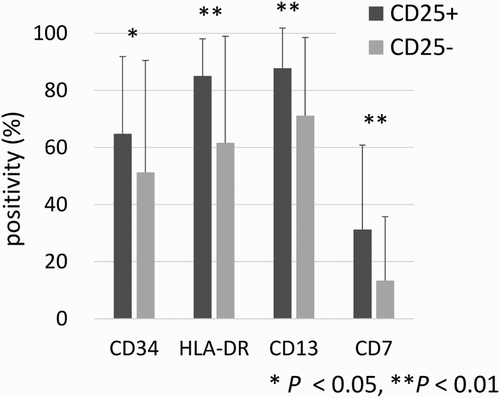
The mean expression of CD34, HLA-DR, CD13, and CD7 was significantly higher in patients with CD25+ AML than in patients with CD25− AML (64.8 vs. 51.3%, p = 0.047; 85.1 vs. 61.7%, p < 0.001; 87.8 vs. 71.2%, p < 0.001; 31.3 vs. 13.4%, p = 0.008, respectively) (). there was no significant difference, low expression of cytoplasmic myeloperoxidase and high expression of cytoplasmic Terminal deoxynucleotidyl transferase (TdT) were observed in CD25+ AML patients compared with CD25− AML patients (64.7 vs. 76.2%, p = 0.22; 21.4 vs. 10.9%, p = 0.24; respectively). Gene mutation analysis, including for FLT3-ITD mutation, was performed in only one patient with CD25+ AML. He did not show FLT3-ITD mutation.
Therapeutic response and prognosis of elderly patients with CD25+ AML
Although this study included 13 patients with APL in the CD25− AML group, analyses of treatments and survival were performed, with the exception of the APL patients. There was no significant difference among treatments for patients with CD25+ AML and CD25− AML. Of 104 patients who underwent induction chemotherapy, 57 patients (54.8%) achieved CR (48 after the first course of induction chemotherapy and 9 after the second course of induction chemotherapy). CR rate was significantly lower in patients with CD25+ AML (n = 15) than those with CD25− AML (n = 89) (20 vs. 60.2%, p = 0.0034). After the first course of induction chemotherapy, there was a significant difference in CR rate by anthracycline and Ara-C regimen between patients with CD25+ AML (n = 8) and those with CD25− AML (n = 55) (0 vs. 63.6%, p = 0.0012). On the other hand, CR rate by CAG regimen did not differ between patients with CD25+ AML (n = 6) and those with CD25− AML (n = 32) (50 vs. 31.3%, p = 0.67). After the second course of induction chemotherapy, none of the patients with CD25+ AML (n = 5) achieved CR, while nine of 14 patients with CD25− AML achieved CR.
In this study, leukocyte counts were not associated with CR rate for any of the cut-off values of 20 000, 25 000, 50 000, or 100 000. CD25+ AML was associated with several poor prognostic factors, such as poor PS and prior hematological diseases, we evaluated whether CR rate could be influenced by other factors, such as poor PS, unfavorable cytogenetics, age of more than 75 years, and secondary AML. Multiple analysis showed that CD25 positivity was an independent predictive factor of induction failure (hazard ratio (HR) 5.2, 95% confidential interval (CI) 1.08–25.3, p = 0.039).
Prognosis of elderly patients with CD25+ AML
During a median follow-up period of 5.4 years, CD25+ AML was associated with an inferior EFS compared with CD25− AML (1-year EFS, 0 vs. 28.4%, respectively, p < 0.001) ((a)). Multivariate analysis using significant factors from a univariate analysis showed CD25 positivity to be a significant risk factor for EFS (HR: 2.1, 95% CI: 1.1–4.3; p = 0.035) ().
Figure 3. Kaplan–Meier estimates of event-free (A) and overall (B) survival in patients with CD25-positive (CD25+) and CD25-negative (CD25−) non-APL AML. APL: acute promyelocytic leukemia; n: number.
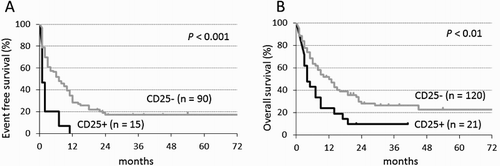
Table 2. Univariate and multivariate analysis for event free survival.
CD25+ AML was also associated with an inferior OS compared with CD25− AML (2-year OS, 9.5 vs. 28.1%, respectively, p < 0.01) ((b)), but multivariate analysis, including cytogenetic risk, age, disease status, and PS factors did not show a significant prognostic value of CD25 positivity for OS (HR: 1.3, 95% CI: 0.7–2.3; p = 0.39).
Subgroup analysis in patients with intermediate cytogenetic risk (n = 89) showed CD25 positivity to be significantly associated with inferior EFS and OS (p < 0.0001, p < 0.0001, respectively). In this subgroup, multivariate analysis, including age, PS, and disease status factors, showed CD25 positivity to be an independent prognostic factor for EFS and OS (HR: 5.5, 95% CI: 2.3–12.9; p < 0.0001; HR: 3.0, 95% CI: 1.4–6.4; p = 0.0049, respectively).
Discussion
In this study, we demonstrated that CD25 expression was associated with adverse clinical outcomes in elderly patients with AML, as well as in young patients. CD25+ AML blasts are characterized by chemotherapy resistance. In previous studies of elderly patients with AML, standard induction chemotherapy showed a CR rate of approximately 50% [Citation2–4]. In this study, overall CR rate was in agreement with previous findings; however, in elderly patients with CD25+ AML, the CR rate was remarkably low and CR could not be achieved by intensive induction chemotherapy consisting of anthracycline and cytarabine. Multivariate analysis showed that CD25 expression had an independent negative impact on the CR rate.
The CR rate of patients with CD25+ AML that received induction therapy by CAG regimen was 50%. These patients were characterized by median bone marrow blasts of 25.3% (range 19–52.8%) and median WBC counts of 5500 (range 1200–9300). Using a mouse model of human AML, LSCs were found to be within the osteoblast-rich area of the bone marrow cell and cycle quiescent status. These LSCs can be induced to enter the cell cycle by G-CSF, and when combined with cell cycle-dependent chemotherapy can induce their apoptosis [Citation7]. Considering the chemotherapy resistance and LSCs-like features in patients with CD25+ AML, a CAG regimen treatment may be effective in elderly CD25+ AML patients with low blasts.
Recently, molecular mutation and gene deregulation have been shown to play an important role in the prognosis of elderly patients with AML. As a relationship between CD25 expression and FLT3-ITD mutation has been reported in young patients with AML [Citation8,Citation10], CD25 expression may be a surrogate marker for FLT3-ITD mutation. Our study was retrospective and FLT3-ITD mutation analysis was not available for most patients. Only one patient with CD25+ AML showed no FLT3-ITD mutation. Further studies are needed to investigate the relationship between FLT3-ITD mutation and CD25 expression in elderly patients with AML.
In this study, an increased expression of CD25 was associated with overt leukemic and relapse statuses. It is unclear whether CD25 expression increased following the progression of the disease or whether small original CD25-positive clones selectively evolved. CD25 is expressed on CD34-positive cells in some patients with late-stage MDS and is associated with azacitidine treatment failure [Citation15]. Additionally, it is speculated that CD25+ myeloblasts at a MDS stage may persist due to chemoresistant properties and expand during the AML transformation. Further evaluation of the biological role of CD25 in clonal evolution is necessary.
Due to a low CR rate, elderly patients with CD25+ AML showed significantly inferior EFS and OS than those with CD25− AML. Multivariate analysis showed CD25 positivity to be a new independent prognostic marker for EFS, distinct from known adverse prognostic markers. There was found to be no significant difference in OS; this may be explained by high mortalities in elderly AML patients compared with young AML patients. In patients with intermediate cytogenetic risk, CD25 positivity was an independent prognostic marker for both EFS and OS. These findings suggest that the evaluation of CD25 expression could be useful for predicting the clinical outcomes of elderly patients with AML.
The previous study showed that CD25 expression had a prognostic value for CR rate and OS in AML patients ≤60 years, but these findings were not observed in older patients [Citation11]. The difference in the results between the previous and current studies may have been due to several reasons. First, the mean level of CD25 expression (sites/cell) was used as a cut-off level in the previous study, while CD25 positivity >10% was used in the current study. The definition of ‘CD25+ AML’ was also different in the two studies. Second, the study period was different in the two studies (1987–2000 in the former vs. 2005–2013 in the latter). Advances in induction therapies, supportive care, allogeneic SCT, etc. could have affected the clinical outcomes. Third, compared with the previous study, the current one included a significant number of secondly and very elderly AML. The different patient characteristics in the two studies may have led to the discrepancies in the results.
A treatment strategy for CD25+ AML has yet to be established in both young and elderly patients. Induction therapy of high-dose daunorubicin followed by autologous SCT did not provide a clinical benefit in young patients with CD25+ AML [Citation8]. Additionally, allogenic SCT did not ameliorate the adverse impact of CD25 expression on OS in AML patients with cytogenetically intermediate risk [Citation16]. In our study, no patients with CD25+ AML received allogenic SCT due to induction failure. Although reduced intensity allogenic SCT for elderly AML patients shows a potential benefit in selected patients [Citation17], there was no clear evidence for the use of SCT in elderly AML patients. If elderly patients with CD25+ AML could achieve CR and be candidates for allogenic SCT treatment, they should promptly receive allogenic SCT at the first CR status using reduced intensity conditioning settings. Additional studies are needed to verify the efficacy of allogenic SCT in elderly patients with CD25+ AML.
In conclusion, we demonstrated for the first time that CD25 expression is an independent adverse prognosis factor in elderly patients with AML. The evaluation of CD25 expression by FCM is useful for predicting clinical outcomes in elderly AML patients. The development of new treatment strategies for elderly patients with CD25+ AML is warranted.
Acknowledgments
The authors would like to thank all of the laboratory technicians in the Division of Cell Transplantation and Transfusion, Jichi Medical University Hospital, who performed FCM.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Shin-ichiro Fujiwara is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Kazuo Muroi is a faculty member at Jichi Medical University, Tochigi, Japan. He is a professor of cell transplantation and transfusion.
Chihiro Yamamoto is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Kaoru Hatano is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Kiyoshi Okazuka is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Kazuya Sato is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Iekuni Oh is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Ken Ohmine is a faculty member at Jichi Medical University, Tochigi, Japan, and expert in the field of hematology.
Takahiro Suzuki is a faculty member at Jichi Medical University, Tochigi, Japan. He is an assistant professor of hematology.
Keiya Ozawa is a faculty member at Jichi Medical University, Tochigi, Japan. He is a professor of hematology.
Additional information
Funding
References
- Thein MS, Ershler WB, Jemal A, et al. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 2013;119(15):2720–2727. doi: 10.1002/cncr.28129
- Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014;32(24):2541–2552. doi: 10.1200/JCO.2014.55.1564
- Nazha A, Ravandi F. Acute myeloid leukemia in the elderly: do we know who should be treated and how?. Leuk Lymphoma. 2014;55(5):979–987. doi: 10.3109/10428194.2013.828348
- Yanada M, Naoe T. Acute myeloid leukemia in older adults. Int J Hematol. 2012;96(2):186–193. doi: 10.1007/s12185-012-1137-3
- Olejniczak K, Kasprzak A. Biological properties of interleukin 2 and its role in pathogenesis of selected diseases--a review. Med Sci Monit. 2008;14(10):Ra179–Ra189.
- Kuhn DJ, Dou QP. The role of interleukin-2 receptor alpha in cancer. Front Biosci. 2005;10:1462–1464. doi: 10.2741/1631
- Saito Y, Kitamura H, Hijikata A, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2(17):170ra9. doi: 10.1126/scitranslmed.3000349
- Gonen M, Sun Z, Figueroa ME, et al. CD25 expression status improves prognostic risk classification in AML independent of established biomarkers: ECOG phase 3 trial, E1900. Blood. 2012;120(11):2297–2306. doi: 10.1182/blood-2012-02-414425
- Terwijn M, Feller N, van Rhenen A, et al. Interleukin-2 receptor alpha-chain (CD25) expression on leukaemic blasts is predictive for outcome and level of residual disease in AML. Eur J Cancer. 2009;45(9):1692–1699. doi: 10.1016/j.ejca.2009.02.021
- Cerny J, Yu H, Ramanathan M, et al. Expression of CD25 independently predicts early treatment failure of acute myeloid leukaemia (AML). Br J Haematol. 2013;160(2):262–266. doi: 10.1111/bjh.12109
- Nakase K, Kita K, Kyo T, et al. Prognostic Relevance of cytokine receptor expression in acute myeloid leukemia: interleukin-2 receptor alpha-chain (CD25) expression predicts a poor prognosis. PLoS One. 2015;10(9):e0128998. doi: 10.1371/journal.pone.0128998
- Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–2333.
- Yamada K, Furusawa S, Saito K, et al. Concurrent use of granulocyte colony-stimulating factor with low-dose cytosine arabinoside and aclarubicin for previously treated acute myelogenous leukemia: a pilot study. Leukemia. 1995;9(1):10–14.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036
- Miltiades P, Lamprianidou E, Vassilakopoulos TP, et al. Expression of CD25 antigen on CD34+ cells is an independent predictor of outcome in late-stage MDS patients treated with azacitidine. Blood Cancer J. 2014;4:e187. doi: 10.1038/bcj.2014.9
- Ikegawa S, Doki N, Yamamoto K, et al. Clinical impact of CD25 expression on outcomes of allogeneic hematopoietic stem cell transplant for cytogenetically intermediate-risk acute myeloid leukemia. Leuk Lymphoma. 2015;56(6):1874–1877. doi: 10.3109/10428194.2014.974044
- Koreth J, Aldridge J, Kim HT, et al. Reduced-intensity conditioning hematopoietic stem cell transplantation in patients over 60 years: hematologic malignancy outcomes are not impaired in advanced age. Biol Blood Marrow Transplant. 2010;16(6):792–800. doi: 10.1016/j.bbmt.2009.12.537