ABSTRACT
Objectives: Recent advancements have promoted the use of T2* magnetic resonance imaging (MRI) in the non-invasive detection of iron overload in various organs for thalassemia major patients. This study aims to determine the iron load in the heart and liver of patients with thalassemia major using T2* MRI and to evaluate its correlation with serum ferritin level and iron chelation therapy.
Methods: This cross-sectional study included 162 subjects diagnosed with thalassemia major, who were classified into acceptable, mild, moderate, or severe cardiac and hepatic iron overload following their T2* MRI results, respectively, and these were correlated to their serum ferritin levels and iron chelation therapy.
Results: The study found that 85.2% of the subjects had normal cardiac iron stores. In contrast, 70.4% of the subjects had severe liver iron overload. A significant but weak correlation (r = −0.28) was found between cardiac T2* MRI and serum ferritin, and a slightly more significant correlation (r = 0.37) was found between liver iron concentration (LIC) and serum ferritin.
Discussion: The findings of this study are consistent with several other studies, which show that patients generally manifest with liver iron overload prior to cardiac iron overload. Moreover, iron accumulation demonstrated by T2* MRI results also show a significant correlation to serum ferritin levels.
Conclusion: This is the first study of its kind conducted in Indonesia, which supports the fact that T2* MRI is undoubtedly valuable in the early detection of cardiac and hepatic iron overload in thalassemia major patients.
Background
Thalassemia major (TM) is a genetic disorder of hemoglobin synthesis that results in significant anemia. Without red blood cell transfusions, most patients with thalassemia would die by the age of 10 [Citation1]. The excess iron acquired from the transfusions can progressively accumulate in various organs, especially the liver, heart, and endocrine organs [Citation2]. Initially, the liver loads with iron. However, once the capacity of transferrin to take up excess iron is surpassed, free iron appears (non-transferrin bound iron NTBI) and begins to enter other organs through alternate pathways aside from the transferrin receptors. Initially, the cells may utilize the excess iron by using it for enzymes and mitochondria, as well as to store ferritin, but if this continues, ultimately the cellular capacity to utilize the free iron will be overwhelmed. Moreover, as intracellular free iron (labile cellular iron – LCI) is toxic, tissue damage may also occur, leading to morbidity and mortality [Citation3]. Humans have no active mechanism to excrete the excess iron from the body. Iron chelation therapy (ICT) is therefore essential for the excretion of excess tissue iron and reducing morbidity and mortality.
Cardiac problems, such as heart failure and arrhythmia, account for up to 71% of all death in thalassemia major patients, and is therefore the leading cause of mortality [Citation4]. In addition, liver fibrosis due to iron overload can be found in 30% of patients [Citation5]. Mortality due to cardiac complications was 46% in our center until 2013.
Measuring tissue iron, total body iron, and adjusting iron chelation therapy appropriately is crucial to patient management. The different methods of evaluating iron profile all have some advantages and disadvantages. Serum ferritin is widely used as a surrogate marker for iron overload. However, it is also an acute phase protein, and hence its levels can be influenced by inflammation, the use of chelation therapy, infection, vitamin C levels, and liver damage [Citation6,Citation7]. Until recently, liver biopsy was considered the gold standard to detect iron overload. However, it is invasive and is associated with several complications. It can also be erroneous due to the heterogeneous distribution of iron throughout the liver, and is not suitable for related long-term follow-up.
Recently, biopsy is being replaced by magnetic resonance imaging techniques. T2* MRI can measure the concentrations of iron in the liver and the heart, and is non-invasive. The result of T2* MRI is particularly beneficial in tailoring the appropriate chelation treatment for each individual patient. In 2012, the Thalassemia Clinical Research Network issued a recommendation stating that T2* imaging of liver and heart should be performed at least annually, beginning at the age of 10 years old. The imaging may be performed more frequently in patients with dangerous levels of cardiac iron as demonstrated by T2* values <10 or excess levels between 10 and 20 ms [Citation8]. Another study in China suggested that MRI evaluation of iron be performed from about 6 years of age [Citation9], and a USA study recommends that it should be conducted even earlier [Citation10].
The objective of this study is to evaluate cardiac and liver iron overload in TM patients in Thalassemia Center at Dr Cipto Mangunkusumo Hospital, Jakarta, Indonesia based on T2* MRI results, and to determine the relationship of these values with their serum ferritin level and the chelation therapy provided.
Methods
Until October 2016, there were 1570 thalassemia patients registered in the Thalassemia Center at Dr. Cipto Mangukusumo Hospital, Jakarta. Most of the patients (75%) were prescribed with 75 mg/kg/d of deferiprone (DFP), and the remaining patients were prescribed with either 25 mg/kg/d of deferasirox (DFX) or 40 mg/kg/d of desferrioxamine (DFO) , four to five times per week, or a combination of 75 mg/kg/d of DFP 75 mg/kg/d, seven times per week + 30 mg/kg/d of DFO, twice a week.
This cross-sectional study was performed on 162 TM subjects. Any patient requiring regular blood transfusions in our unit is regarded as a TM patient. All patients were treated with regular blood transfusion and iron chelation therapy. The cardiac function was evaluated using echocardiography by a cardiologist blinded to clinical information, in particular the chelation therapy. The cardiac iron load was determined by T2* MRI, and the liver iron concentration was calculated from R2* (the inverse of T2*) according to the Wood formula (see below) [Citation11]. The ethical approval was this study was granted by the Faculty of Medicine, Universitas Indonesia prior to conducting the study.
Serum ferritin level
Because of its ease of access, serum ferritin level was performed to evaluate iron overload, potential complications from the iron, and monitor patients’ compliance. The serum ferritin level principally demonstrated purported trends in iron loading. An amount of 3 mL of blood was obtained from each patient for serum ferritin evaluation. Its concentration was analyzed using electrochemiluminescence immunoassay (ECLIA) method with a Cobas 601 machine (Roche Diagnostic).
Echocardiography
Conventional echocardiography was performed at rest by senior fellows in the Pediatric Cardiology Clinic, Department of Child Health, Universitas Indonesia. The parameters tested were systolic function (fractional shortening and ejection fraction derived from fractional shortening through m-Mode echo view automatic calculation) and diastolic function (ratio of the early (E) to late (A) ventricular filling velocity). Normal systolic function was defined as ejection fraction >55% and fraction shortening >27%; while normal dyastolic function was defined as E/A ratio <1.
Cardiac and liver MRI
T2* MRI was performed in Department of Radiology, Universitas Indonesia, using 1.5 Tesla MRI scanner (Siemens Avanto Germany). Myocardial T2* was analyzed using dedicated software (Thalassemia-Tools; Cardiovascular Imaging Solutions, London, United Kingdom) with regions of interest in the ventricular septum which avoid susceptibility artifact. The MRI T2* of the liver was determined using a single 10 mm slice through the center of the liver, which was scanned at 12 different echo times (TE). The TE used was 1.3–23 ms. Each image was acquired during an 11–13 s breath-hold, using a gradient-echo sequence. The repetition time (TR) was 200 ms, the flip angle used was 20°, the base resolution matrix was 128 pixels, the field of view was 39.7 cm × 19.7 cm, and the sampling bandwidth was 125 kHz. The gradient-echo (R2*) and the spin-echo (R2) images were fit to monoexponential equations with a variable offset: S(TE) = Ae–TE × R2* + C. The constant, C, was necessary to compensate for contributions from instrumentation noise and effects from iron-poor species such as blood and bile duct.
Results of cardiac T2* were categorized as severe (T2* < 10 ms), moderate (10 < T2* < 14 ms), mild (14 < T2* < 20 ms), and acceptable (T2* > 20 ms) myocardial involvement [Citation12,Citation13]. The results of liver T2* were converted to liver iron concentration (LIC) in mg/g using the equation (0.0254 × R2*) + 0.202, where R2* is 1000/T2*. Acceptable liver iron was defined as LIC <3.5 mg/g, while mild, moderate, and severe were 3.5–7.0, 7.0–12.0, and >12.0, respectively [Citation11].
Statistical analysis
The data analysis was conducted using SPSS 20 (ChicagoSPSS, SPSS Inc., Chicago, IL) and GraphPad Prism 6. Comparison of cardiac T2* and LIC value among iron chelator groups was performed with Kruskal–Wallis test. Spearman test was used to correlate between serum ferritin level and cardiac T2*and LIC. A p-value of <0.05 was considered statistically significant.
Results
The subjects consisted of 78 (48.1%) males and 84 (51.9%) females. The median age was 14 (3–43) years old. Homozygous β-thalassemia was most common type, which was found in 119 of our subjects (73.5%), followed by 43 with double heterozygous β-thalassemia/HbE (26.5%). Most of the patients received ICT and DFP (71.6%), and a lesser percentage were treated with DFX (14.8%), DFO (6.2%), or alternating combination of DFO and DFP (7.4%). The median serum ferritin level was 3793 (245.6–12 456) ng/mL. Most of the subjects (69.1%) had serum ferritin levels greater than 2500 ng/mL.
Only 50 of the 162 subjects had echocardiography results. The mean of EF, FS, and E/A ratio were 65.3 ± 5.56, 36.6 ± 4.6, and 1.57 ± 0.29, respectively. All subjects had normal systolic and diastolic function. Statistical analysis showed no significant correlation of systolic and diastolic function between the two groups, normal and abnormal cardiac T2* ().
Table 1. Echocardiography profiles among myocardial iron overload groups.
The median cardiac T2* was 34.4 (3.3–76.0) ms. The prevalence of normal myocardial iron based on the results of cardiac T2* was 85.2%. Among 24 (14.8%) subjects with excess myocardial iron, 12 (50%) had mild, 4 (16.7%) moderate, and 8 (33.3%) severe myocardial iron loading. The mean LIC was 15.5 ± 6.5 mg/g dry weight. In contrast, there were no subjects with normal LIC. Most subjects had severe liver iron involvement (70.4%), while a smaller proportion had mild (15.4%) and moderate (14.2%) liver iron involvement. There was no significant correlation between LIC and cardiac T2* (p = 0.038, r = −0.163). Cardiac T2* values showed no relationship with age. The youngest subject with an abnormal cardiac T2* result was 8 years old.
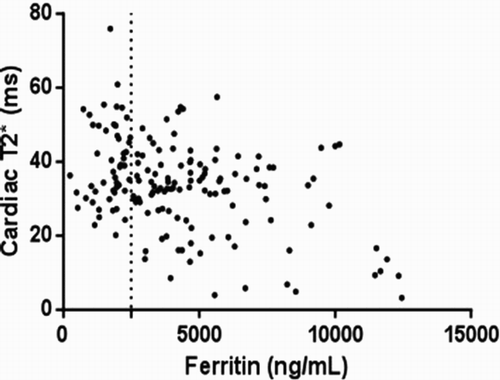
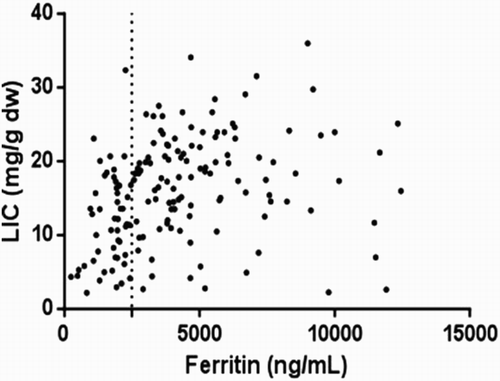
There was a significant but weak correlation (r = −0.28) between cardiac T2* and serum ferritin (), which showed a large variability, confirming that the relation was too weak to be useful in the clinical setting. There was also a significant correlation between LIC and serum ferritin, with a slightly higher clinical significance (, r = 0.37).
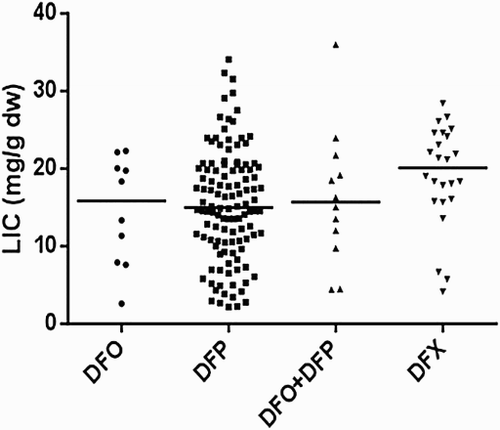
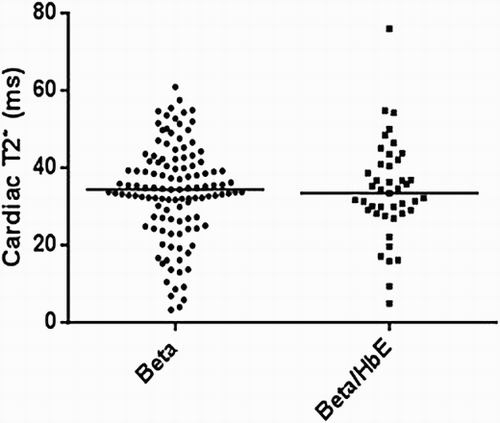
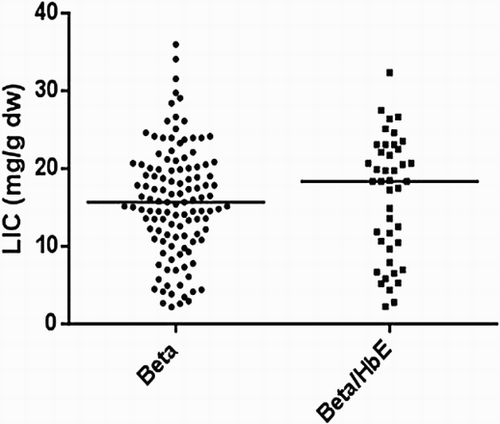
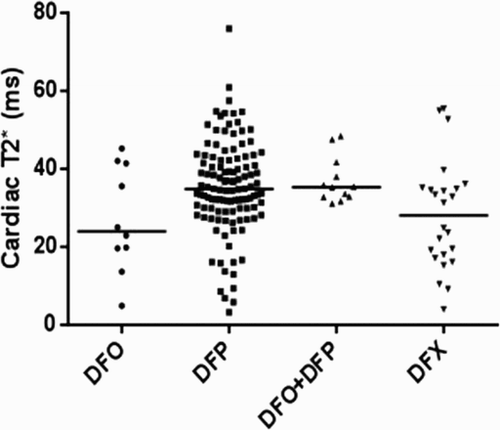
On comparing the cardiac T2* values in a cross-sectional analysis of the four iron chelator groups (), there were significant differences between groups with DFP and the combination of DFP + DFO having the highest cardiac T2* (lowest cardiac iron). In addition, the DFP group had the lowest LIC levels compared to other groups (). There were also no significant differences in cardiac T2* and LIC between beta and beta/HbE thalassemia ( and ).
Figure 3. Comparison of cardiac T2* among chelation groups (p = 0.027). Solid lines showed median levels.
Discussion
This cross-sectional analysis of cardiac and liver iron loading showed that the majority of our patients had acceptable cardiac iron and all of them had liver iron overload. Cardiac and liver iron load showed no relationship with age. However, there was a significant but weak correlation with serum ferritin level, confirming other studies which reported that there was little clinical relevance between cardiac and hepatic iron load and ferritin. One of the notable yet disturbing features found was that four of the children less than 10 years of age had excess cardiac iron.
Regardless of the fact that that ferritin is not so well correlated to the total body iron load [Citation14], as well as the iron load of the various organs of the body, it is still believed that following trends is useful to provide some evaluation with regards to the efficacy of treatment as well as patients’ adherence.
The range of serum ferritin levels in this study varied widely from 100 to more than 10 000 ng/mL. Since most of the patients had a high serum ferritin level, it indicated that poor acceptance of ICT and lack of availability of iron chelators remain the greatest obstacle to iron control. Serum ferritin levels >2500 ng/mL has been suggested as a risk factor associated with an increased risk of heart disease, liver cirrhosis, impaired growth, and delayed puberty [Citation15–17]. Other than the lack of availability and poor compliance in some patients, the incidence of infection among TM patients in our center remain quite high, which may also contribute to a higher ferritin level. Nonetheless, the data from this study confirms those of other studies indicating that ferritin does not necessarily reflect iron overload in body organs, particularly the heart.
Echocardiography is another method to identify the effects of cardiac iron overload. However, this eventually depends on operator expertise. All patients with abnormal cardiac T2* value had a high ejection fraction and good diastolic function. Those normal values could be interpreted as normal cardiac function. This supports the concept that conventional echocardiography is not sufficiently sensitive to pick up cardiac dysfunction and therefore does not permit early intervention [Citation18]. Several studies concluded that tissue Doppler echocardiography was better than conventional echocardiography to distinguish cardiac dysfunction [Citation19,Citation20], and also correlates significantly with cardiac T2* [Citation21]. Diastolic dysfunction generally occurs before systolic dysfunction. A previous study in Jakarta found 84.2% diastolic dysfunction in subclinical patients with tissue Doppler echocardiography parameters. Diastolic parameters can also be a sensitive method to identify early cardiac involvement when patients do not present with the clinical features of cardiac failure [Citation22]. A problem found in our center is that physicians generally do not conduct echocardiography if there are no symptomatic complaints.
Currently, the best method to detect iron overload is T2* MRI. The incidence of abnormal cardiac T2* (<20 ms) among the subjects was lower than that mentioned in other published studies from other countries. A study in Hong Kong found 50% abnormal cardiac T2* [Citation23], and another study in Iran obtained a value of 58% [Citation24]. Our study showed that serum ferritin level had statistically significant correlation with liver and cardiac T2*, and as previously stated, this had little clinical value to determine cardiac and hepatic risk. In addition, serum ferritin may be useful for evaluating adherence and trends over time, as well as to offer encouragement to patients. Eghbali et al. [Citation25] found a correlation between serum ferritin level and liver T2*, but not cardiac T2*.
Although T2* MRI could substitute serum ferritin and biopsy, this test is expensive and there is currently only very limited equipments set up to perform it in Indonesia. Therefore, serum ferritin remains the primary screening tool in developing countries, and it should be tested every 3 months following ferritin trends, based on the International Thalassaemia Federation guidelines.
The correlation between LIC and cardiac T2* was weak in this study [r = −0.163]. This is similar to other studies which found no correlation between the two variables [Citation13,Citation26–28]. The magnitude of liver iron overload does not correlate with myocardial iron. This means that although the level of iron overload in the liver was severe, the myocardial iron overload could still be normal or mildly increased. As previously discussed, this can be explained by the fact that that the iron obtained from blood transfusion is not reused for erythropoiesis, and will primarily saturate initially in the reticulo-endothelial system of the liver, spleen and bone marrow, after which free iron will appear in the circulation. Iron will begin to accumulate in the heart and other organs once this occurs [Citation29].
The median levels of T2* cardiac and LIC varied between chelator regimes. This study showed differences in cardiac and liver iron overload according to the chelation regimens. Several studies indicated that DFP is superior for myocardial iron removal compared to DFO [Citation30], and is also linked with improved survival [Citation31–33]. Another prospective study in Taiwan found that DFP was more beneficial in improving cardiac function compared to DFO, but there were no statistically significant differences between the two groups in terms of reducing serum ferritin levels and hepatic iron overload [Citation33].
An important aspect that can be considered from these results is the changes in the iron chelation regimen received by the subjects, which can be modified either by the type of iron chelation treatment, the dose, or the combination, in accordance to that which would best suit the subjects. However, changing treatment regimens, particularly in developing countries, can be difficult due to financial issues and the limited coverage of the national insurance. A reasonable alternative that has been attempted is to maximize the use of monotherapy or provide alternating combination therapy, which have shown promising results. The provision of psychological support and education to patients and family members are extremely important and have shown benefits in terms of compliance to treatment.
Our patients generally preferred DFP compared to DFO due to its oral administration. Both its acceptability and better efficacy in removing cardiac iron can explain the reason for the low incidence of excess cardiac iron among our patients. Meanwhile, the availability of DFX is very limited and the optimal doses cannot be provided due to the high cost. Subjects showed less iron overload, especially in the liver, if the dose of DFP and DFX are increased to 100 and 40 mg/kg, respectively.
The youngest patient who had an abnormal cardiac T2* result was 8 years old. A study in China reported three patients aged 6 years old who developed severe cardiac iron overload, and another study from USA also showed cardiac iron load in TM patients before the age of 10 years. This further confirms that cardiac T2* should be performed early, even though it will require adequate cooperation from the child, since T2* MRI in our center is performed without sedation. In the USA, patients may be sedated for MRI, but this is regarded as being relatively invasive and has not been accepted universally [Citation10].
In conclusion, a large majority of the subjects were found to have high iron overload, indicating a need to re-evaluate the iron chelation therapy being received and ensuring the optimal tailored dosage for each individual patient. This study found that hepatic and cardiac MRI were extremely valuable in determining total body iron and specific organ iron overload early, even within the context of significantly limited resources. Since serum ferritin did not prove to be an adequate measure of iron overload in these patients, it is important to ensure that iron load assessments by T2* MRI are made more accessible to the patients, and these results should be used to encourage patients to take their chelation therapy and tailor it to reduce their iron load and thereby the risks of morbidity and mortality.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr. Pustika Amalia Wahidiyat is a pediatric hematologist-oncologist at Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. She is an expert in the field of hematology with a special interest in thalassemia.
Dr. Felix Liauw is a general pediatrician who graduated from the Faculty of Medicine, Universitas Indonesia and completed his pediatric residency training at Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia.
Dr. Damayanti Sekarsari is a radiologist at Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. She is an expert in the field of pediatric radiology.
Ms. Siti Ayu Putriasih is a psychologist at the Thalassemia Center, Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia, with a special interest in clinical psychology.
Dr. Vasili Berdoukas is a pediatric hematologist currently residing in China. He is an expert in the field of hemoglobinopathies and has various publications in the field.
Prof. Dudley J. Pennell is a cardiologist and the director of the cardiovascular biomedical research unit (BRU) and the cardiovascular magnetic resonance unit (CMR) at the Royal Brompton Hospital, London, United Kingdom.
ORCID
Pustika Amalia Wahidiyat http://orcid.org/0000-0002-5513-006X
References
- Cucchi P, Vullo C, Barrai I. Population genetics in the province of ferrara. II. Survival of children with Cooley’s anemia. Am J Hum Genet. 1977;29:178–183.
- Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–761.
- Cabantchik ZI, Breuer W, Zanninelli G, et al. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003
- Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107:3733–3737. doi: 10.1182/blood-2005-07-2933
- Li CK, Chik KW, Lam CWK, et al. Liver disease in transfusion dependent thalassaemia major. Arch Dis Child. 2002;86:344–347. doi: 10.1136/adc.86.5.344
- Voskaridou E, Douskou M, Terpos E, et al. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol. 2004;126:736–742. doi: 10.1111/j.1365-2141.2004.05104.x
- Mavrogeni SI, Markussis V, Kaklamanis L, et al. A comparison of magnetic resonance imaging and cardiac biopsy in the evaluation of heart iron overload in patients with beta-thalassemia major. Eur J Haematol. 2005;75:241–247. doi: 10.1111/j.1600-0609.2005.00474.x
- The Cooley’s Anemia Foundation. Position statement on MRI based hepatic iron assessment methods. http://www.thalassemia.org/2012-position-statement-on-mri-based-hepatic-iron-assessment-methods-2. Accessed September 13, 2015.
- Yang G, Liu R, Peng P, et al. How early can myocardial iron overload occur in beta thalassemia major? PLoS One. 2014;9:e85379. doi: 10.1371/journal.pone.0085379
- Berdoukas V, Nord A, Carson S, et al. Tissue iron evaluation in chronically transfused children shows significant levels of iron loading at a very young age. Am J Hematol. 2013;88:E283–E285. doi: 10.1002/ajh.23543
- Wood JC, Enriquez C, Ghugre N, et al. MRI r2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982
- Carpenter JP, He T, Kirk P, et al. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123:1519–1528. doi: 10.1161/CIRCULATIONAHA.110.007641
- Di Tucci AA, Matta G, Deplano S, et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemia. Haematologica. 2008;93:1385–1388. doi: 10.3324/haematol.12759
- Puliyel M, Sposto R, Berdoukas VA, et al. Ferritin trends do not predict changes in total body iron in patients with transfusional iron overload. Am J Hematol. 2014;89:391–394. doi: 10.1002/ajh.23650
- Telfer PT, Prestcott E, Holden S, et al. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110:971–977. doi: 10.1046/j.1365-2141.2000.02298.x
- Angulo IL, Covas DT, Carneiro AA, et al. Determination of iron-overload in thalassemia by hepatic MRI and ferritin. Rev Bras Hematol Hemoter. 2008;30:449–452.
- Shalitin S, Carmi D, Weintrob N, et al. Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. Eur J Haematol. 2005;74:93–100. doi: 10.1111/j.1600-0609.2004.00371.x
- Moussavi F, Ghasabeh MA, Roodpeyma S, et al. Optimal method for early detection of cardiac disorders in thalassemia major patients: magnetic resonance imaging or echocardiography? Blood Res. 2014;49:182–186. doi: 10.5045/br.2014.49.3.182
- Balci YI, Gurses D. Detection of early cardiac dysfunction in patients with β-thalassemia major and thalassemia trait by tissue Doppler echocardiography. Pediatr Hematol Oncol. 2011;28:486–496. doi: 10.3109/08880018.2011.568596
- Silvilairat S, Sittiwangkul R, Pongport Y, et al. Tissue Doppler echocardiography reliably reflects severity of iron overload in pediatric patients with β thalassemia. Eur J Echocardiogr. 2008;9:368–372.
- Aypar E, Alehan D, Hazirolan T, et al. The efficacy of tissue Doppler imaging in predicting myocardial iron load in patients with beta-thalassemia major: correlation with T2* cardiovascular magnetic resonance. Int J Cardiovasc Imaging. 2010;26:413–421. doi: 10.1007/s10554-010-9591-6
- Josep R, Wahidiyat PA, Trihono PP, et al. Comparison of cardiac dysfunction in thalassemia major patients using deferoxamine or deferiprone as an iron-chelating agent. Paediatr Indones. 2012;52:272–279. doi: 10.14238/pi52.5.2012.272-9
- Leung AWK, Chu WCW, Lam WWM, et al. Magnetic resonance imaging assessment of cardiac and liver iron load in transfusion dependent patients. Pediatr Blood Cancer. 2009;53:1054–1059. doi: 10.1002/pbc.22170
- Majd Z, Haghpanah S, Ajami GH, et al. Serum ferritin levels correlation with heart and liver MRI and LIC in patients with transfusion-dependent thalassemia. Iran Red Crescent Med J. 2015;17:e24959. doi: 10.5812/ircmj.17(4)2015.24959
- Eghbali A, Taherahmadi H, Shahbazi M, et al. Association between serum ferritin level, cardiac and hepatic T2-star MRI in patients with major β-thalassemia. Iran J Ped Hematol Oncol. 2014;4:17–21.
- Perifanis V, Christoforidis A, Vlachaki E, et al. Comparison of effects of different long-term iron-chelation regimens on myocardial and hepatic iron concentrations assessed with T2* magnetic resonance imaging in patients with beta-thalassemia major. Int J Hematol. 2007;86:385–389. doi: 10.1007/BF02983992
- Tanner MA, Galanello R, Dessi C, et al. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8:543–547. doi: 10.1080/10976640600698155
- Aessopos A, Fragodimitri C, Karabatsos F, et al. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica. 2007;92:131–132. doi: 10.3324/haematol.10455
- Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822
- Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo controlled, double blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790
- Telfer P, Coen PG, Christou S, et al. Survival of medically treated thalassemia patients in Cyprus trends and risk factors over the period 1980-2004. Haemotologica. 2006;91:1187–1192.
- Lai ME, Grady RW, Vacquer S, et al. Increased survival and reversion of iron-induced cardiac disease in patients with thalassemia major receiving intensive combined chelation therapy as compared to deferoxamine alone. Blood Cells Mol Dis. 2010;45:136–139. doi: 10.1016/j.bcmd.2010.05.005
- Peng CT, Chow KC, Chen JH, et al. Safety monitoring of cardiac and hepatic systems in β-thalassemia patients with chelating treatment in Taiwan. Eur J Haematol. 2003;70:392–397. doi: 10.1034/j.1600-0609.2003.00071.x