ABSTRACT
Objectives: To investigate the dynamic change of follicular T helper cells (TFH) in patients with malignant lymphoid disease (MLD) and to explore its clinical significance.
Methods: The dynamic change of TFH cells, ICOS+- and PD-1+ TFH cells at pretreatment and different treatment periods was determined by flow cytometry in 85 MLD patients. Concentration of interleukin 21 (IL-21) was evaluated by ELISA, and the correlation between clinical prognosis and the ratio of TFH cells was analyzed.
Results: Significantly increased ICOS+- and PD-1+ TFH cells were found in MLD patients at pretreatment compared to healthy controls. Decreased or even close to normal levels of ICOS+- and PD-1+ TFH cells were found at the end of treatment. However, in the patients with progressive disease, high levels of ICOS+- and PD-1+ TFH cells were found. Moreover, a significantly increased plasma IL-21 level was found in MLD patients. Negative correlation was found between the level of ICOS+-, PD-1+ TFH cells, as well as IL-21 and the prognosis of MLD.
Conclusions: Significantly increased TFH cell ratios were found in patients with MLD, and decreased TFH cells ratios could be expected in those treatment-effective patients, which could be used as the therapeutic efficacy index.
Introduction
Malignant lymphoid diseases (MLDs), including acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM), are malignancies which arise from lymphocytes residing in lymphoid tissue outside of the marrow. Until now, the detailed pathogenesis of MLD has not been fully explored and abnormal immune system is considered to be the main cause of MLD. Moreover, multiple factors, including gene mutation, epigenetic alterations and change of hematopoietic microenvironment, could induce the development and progression of MLD. Understanding the detailed behaviors of immune system could be helpful in the effective treatment of MLD.
Follicular T helper cells (TFH), which are different from Th1, Th2, Th17, and Tregs, are a novel subset of CD4+ T cells located in germinal center (GC) of B cells. The TFH cells display the features of CXCR5, ICOS, PD-1 expression, and IL-21 secretion, which could be further involved in the B-cell activation, antibody production, and humoral immune response [Citation1–3]. The abnormal change of TFH cells has been proved in multiple diseases, including immune disease, rheumatic diseases, immunodeficiency disease, and tumor [Citation4–7].
In the present study, we aimed to investigate the dynamic change of TFH in MLD patients during the treatment process and to explore its clinical significance.
Materials and methods
Subjects
From June 2014 to January 2016, a total of 85 patients treated in the Department of Hematology of our hospital were included in this study. Among these patients, 13 cases were acute lymphoblastic leukemia (ALL), 52 cases were NHL, and 20 cases were multiple myeloma (MM). All the patients were diagnosed according to the diagnosis and treatment criteria of hematological disease. The detailed clinical characteristics of these patients are shown in . We also recruited age-matched health control subjects from the physical examination department and a total of 10 cases were included, including 5 males and 5 females, with a median age of 50 (44–65) years. The Institutional Review Board at the Second Affiliated Hospital of Soochow University approved all protocols, and informed consent was obtained from all adult donors.
Table 1. Clinical characteristics of MLD.
Peripheral blood mononuclear cells and plasma sampling
A total of 4 mL heparin anticoagulant peripheral blood was collected at pretreatment and the end of second, fourth, and sixth treatment course. The plasma used for IL-21 concentration determination was obtained by 3000 rpm centrifugation for 10 min. The remaining blood was diluted with phosphate-buffered saline (PBS) and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation at 2000 rpm for 10 min. Both plasma and PBMCs were stored in −80°C until further experiments.
Flow cytometry analysis of TFH cells
PBMCs were thawed and adjusted to a density of 1 × 106 cells/mL with PBS. One hundred microliter of PBMCs solution was aliquoted and a volume of 10 μL PerCP-CD4 or Isotype IgG and FITC-CXCR5, PE-ICOS, and PE-Cy7-PD1 were added. After incubation for 30 min, the cells were processed in a flow cytometry and CD4+ cells were used for gating. Here, we defined CD4+CXCR5+ cells as TFH cells and further analyses were also performed by detecting the ICOS+ and PD1+ expression. The ratio of peripheral TFH cells was defined the ratio of CXCR5+CD4+ cell to CD4+ cells, whereas the ICOS+- and PD1+ peripheral TFH cells stand for the ratio of ICOS+CXCR5+CD4+ cell to CXCR5+CD4+ cells and PD1+ ICOS+CXCR5+CD4+ cell to CXCR5+CD4+ cells, respectively. All the data were analyzed by the Summit 4.0 software. All the antibodies used here were purchased from eBioscience Co. Ltd.
ELISA
The plasma inflammatory factor IL-21 was measured by quantitative colorimetric sandwich ELISA’s kit (R&D, Minneapolis, MN).
Statistical analysis
All the data were processed with SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. Student’s t-test or one-way analysis of variance was used to examine differences between groups. Multiple linear regression was performed for the correlation analysis. A p-value of <0.05 was considered significant.
Results
Characteristics of the MLD patients
As shown in , there were 5, 31, and 11 males in the groups of ALL, NHL, and MM, respectively. The average ages were 49.6 ± 15.9, 55.8 ± 16.0, and 57.9 ± 11.9 in the three groups, respectively. The numbers of gene mutation including RBB2H, RBB2H and CEBPA, FLT3-TKD and FLT3-ITD, and Karyotypic anomalies which include deletion and malposition are also listed in . Lab tests, including concentration of hemoglobin, platelet, lactate dehydrogenase, and β2-microglobulin, are also recorded in .
Elevated peripheral TFH cells in MLD patients
We observed significantly increased ratio of peripheral TFH cells in MLD patients compared to healthy controls (32.06 ± 8.06% vs. 11.06 ± 0.88%, p < 0.001). Different ratios of peripheral TFH cells in different type of MLDs: NHL (36.04 ± 6.39%) > ALL (30.03 ± 6.07%) > MM (23.01 ± 4.69%), which showed a significant difference among these groups (p < 0.001). Significantly decreased ratio of peripheral TFH cells in those MLD patients who achieved an effective treatment at the end of second, fourth, and sixth treatment course compared to pretreatment (p < 0.05). A similar or even lower ratio of peripheral TFH cells could be observed in MLD patients, especially in those patients with complete remission, at the end of sixth treatment course compared to healthy controls (p > 0.05); however, a higher ratio of peripheral TFH cells was still found in those patients with partial remission (p < 0.05). In addition, a higher ratio of peripheral TFH cells was found in NHL and MM patients with disease progression compared to pretreatment, while slightly lower ratio of peripheral TFH cells was found in ALL patients with disease progression compared to pretreatment. These results suggested that the ratio of peripheral TFH cells of a patient who is treated and responds well reverts towards that in a normal subject.
Elevated ICOS+- and PD1+-peripheral TFH cells were found in MLD patients
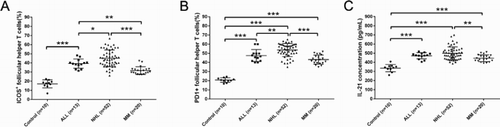
Further analysis showed that the ratio of ICOS+- and PD1+-peripheral TFH cells in healthy controls was 16.08 ± 5.51% and 20.68 ± 2.50%, whereas significantly elevated ICOS+- and PD1+-peripheral TFH cells were found in MLD patients. The ratio of ICOS+- and PD1+-peripheral TFH cells was 39.21 ± 5.04% and 47.52 ± 6.57% in ALL patients, 45.01 ± 9.14% and 53.52 ± 7.16% in NHL patients, and 31.58 ± 4.20% and 43.14 ± 5.01% in MM patients, which showed a statistical difference compared to healthy controls (p < 0.001) ().
Figure 1. Upregulation ratio of ICOS+ and PD+ follicular help T cells (TFH) as well as concentration of IL-21 in patients with MLD. The ratio of TFH was determined by flow cytometry analysis. Plasma IL-21 level was determined by enzyme-linked immunosorbent assay. *p < 0.05, **p < 0.01, and ***p < 0.001 for between-group comparison.
Dynamic changes of ICOS+- and PD1+-peripheral TFH cells during the treatment process
Significantly decreased ICOS+ peripheral TFH cells were found in those patients with complete remission (CR) and partial remission (PR) (p < 0.001). The ratio of ICOS+ peripheral TFH cells at the end of second, fourth, and sixth treatment course was, respectively, 29.59 ± 4.21%, 23.63 ± 4.17%, and 18.07 ± 6.14% in ALL patients, 38.77 ± 4.12%, 27.58 ± 4.22%, and 19.34 ± 5.08% in NHL patients, and 28.18 ± 4.46%, 21.23 ± 5.15%, and 17.55 ± 6.17% in MM patients ((a)). In addition, in those patients with no response or progressive disease, no significant difference was found on the ratio of ICOS+ peripheral TFH cells at the specific treatment time point compared to pretreatment ((a)).
Figure 2. Changes of ratio of ICOS+ and PD1+ follicular help T cells (TFH) as well as concentration of IL-21 in patients with MLD after chemotherapy at second, fourth, and sixth treatment course. The ratio of TFH was determined by flow cytometry analysis. Plasma IL-21 level was determined by enzyme-linked immunosorbent assay. *p < 0.05, **p < 0.01, and ***p < 0.001 for between-group comparison.
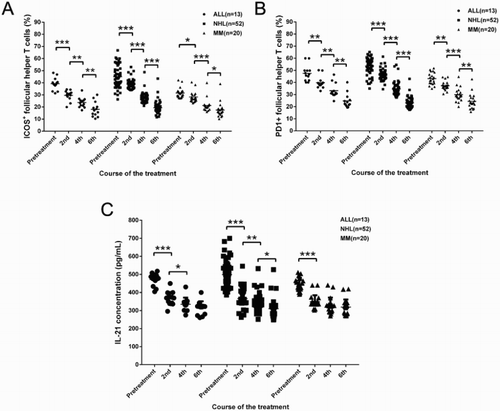
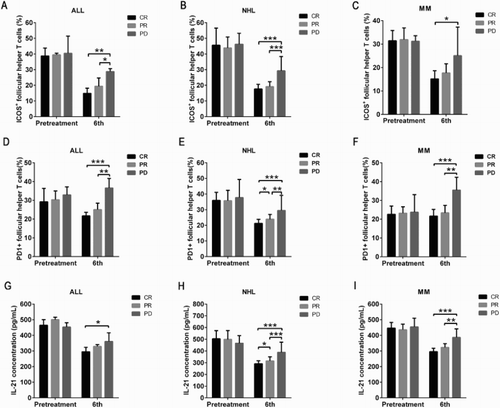
Figure 3. Ratio of ICOS+ and PD1+ follicular help T cells (TFH) as well as concentration of IL-21 among the patients with the prognosis of complete remission (CR), partial remission (PR), and disease progression (PD). (a,d,g) The differences in the ratios of ICOS+ TFH, PD1+ TFH, and concentration of IL-21 in ALL among CR, PR, and PD, respectively. (b,e,h) The differences in the ratios of ICOS+ TFH, PD1+ TFH, and concentration of IL-21 in NHL among CR, PR, and PD, respectively. (c,f,i) The differences in the ratios of ICOS+ TFH, PD1+ TFH, and concentration of IL-21 in multiple myeloma (MM) among CR, PR, and PD, respectively. *p < 0.05, **p < 0.01, and ***p < 0.001 for between-group comparison.
PD1+ peripheral TFH cells showed a similar pattern as ICOS+ peripheral TFH cells, significantly decreased PD1+ peripheral TFH cells were found in those patients at the end of second, fourth, and sixth treatment course (p < 0.05). Ratio of PD1+ peripheral TFH cells in different treatment courses was, respectively, 39.82 ± 5.00%, 33.24 ± 5.91%, and 24.77 ± 5.96% in ALL patients, 46.40 ± 4.53%, 34.66 ± 5.82%, and 23.07 ± 4.49% in NHL patients, and 37.33 ± 3.70%, 29.93 ± 5.56%, and 24.33 ± 6.29% in MM patients ((b)). Similar ratio of PD1+ peripheral TFH cells was found in NHL and MM patients at the end of sixth treatment course, while significantly increased ratio of PD1+ peripheral TFH cells was found in ALL patients compared to healthy controls. In addition, no difference was found on PD1+ peripheral TFH cells in those patients with no response or progressive disease ((b)).
Elevated plasma IL-21 was found in MLD patients
Interleukin (IL)-21, a cytokine, has a fundamental role in the development of T-cell-dependent B-cell responses and, therefore, we determined the plasma IL-21 in all these patients. The plasma IL-21 was 471.40 ± 33.95 pg/mL, 498.74 ± 70.77 pg/mL, and 444.18 ± 37.56 pg/mL in ALL, NHL, and MM patients, respectively, which showed a significant increase compared to that in healthy controls (336.57 ± 40.56 pg/mL, p < 0.001) ((c)). Moreover, significant difference of IL-21 level was found between NHL and MM patients (p < 0.01). Concentrations of IL-21 at the end of second, fourth, and sixth treatment course were, respectively, 364.13 ± 38.27 pg/mL, 334.78 ± 36.22 pg/mL, and 313.38 ± 37.40 pg/mL in ALL patients, 366.27 ± 49.97 pg/mL, 337.20 ± 45.38 pg/mL, and 310.14 ± 46.44 pg/mL in NHL patients, 348.13 ± 36.05 pg/mL, 328.23 ± 43.28 pg/mL, and 319.18 ± 41.52 pg/mL in MM patients ((c)), and significant differences could be found between second and fourth treatment course in ALL patients (p < 0.05), between second and fourth treatment course (p < 0.01) and between fourth and sixth treatment course (p < 0.05) in NHIL patients, and between pretreatment and second treatment course in ALL (p < 0.001), NHL (p < 0.001), and MM (p < 0.001) patients. Similarly, there were no differences between the concentrations of IL-21 in those patients on pretreatment and with no response or progressive disease ((c)).
Correlations between prognosis and the ratio of peripheral TFH cells
We performed the correlation analyses between the prognosis and the ratio of peripheral TFH cells, and the results are shown in . According to the results, negative correlation was found between the prognosis and ratio of PD1+ and ICOS+ peripheral TFH cells. Similarly, negative correlation was found between the prognosis and concentration of IL-21 ().
Table 2. Relationship between changes of surface markers of the follicular help T cells (TFH) and prognosis of MLDs.
Discussion
Early since 1960s, Claman and Miller first proposed the role of T helper cells especially Th2 cells in B-cell activation and antibody production using irradiated or thymectomized mouse models [Citation8,Citation9]. The critical role of Th2 cells in B-cell function mature was emphasized for a long time until the discovery of follicular helper T cells (TFH). Studies have verified TFH cells as the truly functional cells in promoting B-cell proliferation, differentiation, antibody secretion, and involvement in humoral immune response [Citation10–13].
As a novel subpopulation of CD4+ T cells, TFH cells play a critical role in maintaining the immune homeostasis. Previous studies have elucidated the functional role of TFH cells in immune system. The interactions between TFH cells and B cells could be realized via high expression of chemokine receptor CXCR5 on the surface of TFH and the expression of its ligand CXCL13 in the follicular GC of the B cells, which could result in the migration and location of TFH into the GC [Citation14,Citation15]. Moreover, the expression of costimulatory molecule ICOS on the surface of TFH could further induce the production and function maintenance of TFH cells in participating in the generation of GC and memory B cells via interaction with its ligand ICOS-L. Several studies have shown that downregulation expression of ICOS could result in decreasing number of TFH, thereby affecting the mature and class switching of B cells, suggesting the irreplaceable role of ICOS in TFH function [Citation16–18]. Programmed death receptor 1 (PD), which could interact with its ligand PD-L1 to transmit inhibition signaling to affect the T-cell differentiation and Treg production, is another important marker on TFH cells. Blocking the PD-1/PD-L1 signaling could lead to the massive proliferation of TFH cells, thereby resulting in the B-cell proliferation and antibody production [Citation19–21]. IL-21 is a key cytokine involved in the TFH cell production, and it can upregulate the expression of CXCR5 and ICOS through an autocrine manner, thereby promoting the TFH cell differentiation and function development. In addition, IL-21 was also considered to be involved in the GC formation, B-cell proliferation and differentiation, and IgG class switching [Citation22,Citation23].
In the present study, we found that TFH cells, ICOS, PD-1, and IL-21 were participated in the development and progression of MLD. Significantly elevated TFH and the expression of ICOS, PD-1, and IL-21 were found at pretreatment compared to healthy controls, whereas significantly decreased TFH cells, ICOS and PD-1 were found at the end of second, fourth, and sixth treatment course in those patients with CR or PR. However, the decreasing of TFH cells and its related molecules ICOS and PD-1 did not reach to the normal level although most of patients achieved a remission statue. At the end of sixth treatment course, a similar ratio of TFH cells in the MLD patients could be observed, indicating the treatment duration-dependent property of TFH in MLD, which might be served as a prognostic index in clinical practice. Further comparisons between the CR and PR patients revealed that significantly lower level of TFH cells could be expected in CR patients than in PR patients, suggesting a negative correlation between TFH number and therapeutic effect. We also found that significantly increased or similar amount of TFH, ICOS and PD-1 in those patients with no response or progressive disease at the end of second treatment course. Combining with the correlation analysis, we concluded that late staging of patient could result in poor prognosis, which is illustrated by no decrease in the TFH cell number. Moreover, owing to the similar increasing pattern of TFH cells as the disease progress, we proposed a close relationship between TFH cells and MLD, and TFH cells could be employed as a novel indicator in peripheral blood for disease progression. We compared the difference in the TFH cells, ICOS, PD-1, and IL-21 among all the three group of patients, and the results showed the highest level of TFH cells, ICOS, PD-1, and IL-21 in NHL patients and lowest in MM patients. A recent study also indicated the relationship between plasma cells inhibition and TFH. Pelletier et al. [Citation24] demonstrate that isotype-switched plasma cells expressed major histocompatibility complex (MHC) class II, the costimulatory molecules CD80 and CD86, and the intracellular machinery required for antigen presentation. Antigen-specific plasma cells accessed, processed, and presented sufficient antigen in vivo to induce multiple helper T-cell functions. They found that antigen-primed plasma cells failed to induce IL-21 or the transcriptional repressor Bcl-6 in naive helper T cells and actively decreased these key molecules in antigen-activated TFH cells. Mice lacking plasma cells showed altered TFH cell activity, which provided evidence of this negative feedback loop. Hence, antigen presentation by plasma cells defines a previously unknown layer of cognate regulation that limits the antigen-specific TFH cell program that controls ongoing B-cell immunity.
Recent studies have also shown the clinical significance of TFH cells in patients with immune thrombocytopenia (ITP), and they concluded that higher proportion of TFH cells, which could be rectified by hormone therapy, might account for the decreased platelet counts to be further involved in the immunological pathogenesis of children ITP [Citation25,Citation26]. Moreover, Dogan et al. [Citation27] reported that abnormal expression of CXCL13, a chemokine critical for GC formation and one of the most highly upregulated genes in the GC T helper cell subset, in the majority of angioimmunoblastic T-cell lymphoma cases, provided further support for the role of GC T helper cells as the cells of origin for angioimmunoblastic T-cell lymphoma. Battistella et al. [Citation28] identified five cases of cutaneous T-cell lymphoma with a peculiar pathologic aspect and expression of TFH markers CD10 in four of five biopsy specimens further showed medium-sized to large-sized atypical T-cell skin infiltrate expressing TFH markers (CD10, Bcl-6, PD-1, CXCL13, and ICOS), and this is the first report of the presence of TFH in lymphoma. They also proposed to examine the expression of TFH cells to improve prognosis in those patients with no response to CD20 antibody treatment.
On the basis of the elucidated role of TFH cell in some of the diseases and PD-1 was confirmed as the early marker expressed on the TFH cells, we proposed PD-1 as the main marker in tumors and the use of PD-1 blocking therapy might serve as a promising treatment intervention in different types of tumors to improve the overall survival in these patients. Recently, the development of PD-1 monoclonal antibody, such as pembrolizumab, has been employed in the treatment of melanoma and non-small lung cancer and the mechanism includes blocking its interaction with PD-L1 and PD-L2, thereby affecting tumor escaping and activation of immune system [Citation29].
In conclusion, we found that upregulation of TFH cells, ICOS, PD-1, and IL-21 was found in the peripheral blood of MLD patients, which could be decreased to normal level in those patients with response to the treatment. Moreover, a close level of TFH to normal could be expected in CR patients than PR patients, suggesting the involvement of TFH in the progression of MLD. We concluded that consistent level of TFH cells and disease activity could be found in MLD patients and treatment targeting TFH might serve as an efficacy way to improve the disease state of the patients.
Disclosure statements
No potential conflict of interest was reported by the authors.
Notes on contributors
Dong-Ming Zhou is a doctor in the Second Affiliated Hospital of Soochow University, China.
Yan-Xia Xu is a graduate student at Soochow University, majoring in internal medicine.
Li-Ying Zhang is a doctor in the Second Affiliated Hospital of Soochow University, China.
Yu Sun is an assistant professor of Hematology in the Second Affiliated Hospital of Soochow University, China.
Zi-Yan Wang earned her PhD degree in internal medicine from Soochow University, China. Now she works as a doctor in the Second Affiliated Hospital of Soochow University.
Yu-Qing Yuan is an assistant professor of Hematology in the Second Affiliated Hospital of Soochow University, China.
Jin-Xiang Fu is a professor of Hematology in the Second Affiliated Hospital of Soochow University, China. His research focuses on malignant lymphoid disease.
Additional information
Funding
References
- Fazilleau N, Mark L, McHeyzer-Williams LJ, et al. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–35. doi: 10.1016/j.immuni.2009.03.003
- Rodriguez-Pinilla SM, Atienza L, Murillo C, et al. Peripheral T-cell lymphoma with follicular T-cell markers. Am J Surg Pathol. 2008;32(12):1787–99. doi: 10.1097/PAS.0b013e31817f123e
- Nurieva RI, Chung Y. Understanding the development and function of T follicular helper cells. Cell Mol Immunol. 2010;7(3):190–7. doi: 10.1038/cmi.2010.24
- Grammer AC, Slota R, Fischer R, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154–CD40 interactions. J Clin Invest. 2003;112(10):1506–20. doi: 10.1172/JCI200319301
- Zhu C, Ma J, Liu Y, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocr Metab. 2012;97(3):943–50. doi: 10.1210/jc.2011-2003
- Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–44. doi: 10.1002/art.25032
- Bosisio FM, Cerroni L. Expression of T-follicular helper markers in sequential biopsies of progressive mycosis fungoides and other primary cutaneous T-cell lymphomas. Am J Dermatopathol. 2015;37(2):115–21. doi: 10.1097/DAD.0000000000000258
- Claman HN, Chaperon EA, Triplett RF. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966;122(4):1167–71. doi: 10.3181/00379727-122-31353
- Miller JF, Mitchell GF. Cell to cell interaction in the immune response: I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968;128(4):801–20. doi: 10.1084/jem.128.4.801
- Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192(11):1553–62. doi: 10.1084/jem.192.11.1553
- Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192(11):1545–52. doi: 10.1084/jem.192.11.1545
- McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268(5207):106–11. doi: 10.1126/science.7535476
- Linterman MA, Vinuesa CG. T follicular helper cells during immunity and tolerance. Prog Mol Biol Transl Sci. 2010;92:207–48. doi: 10.1016/S1877-1173(10)92009-7
- Bentebibel S-E, Schmitt N, Banchereau J, et al. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci USA. 2011;108(33):E488–97. doi: 10.1073/pnas.1100898108
- Ferretti E, Bertolotto M, Deaglio S, et al. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia. 2011;25(8):1268–77. doi: 10.1038/leu.2011.88
- Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–46. doi: 10.1016/j.immuni.2011.03.023
- Bertino SA, Craft J. Roquin paralogs add a new dimension to ICOS regulation. Immunity. 2013;38(4):624–6. doi: 10.1016/j.immuni.2013.03.007
- Akiba H, Takeda K, Kojima Y, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175(4):2340–8. doi: 10.4049/jimmunol.175.4.2340
- Hams E, McCarron MJ, Amu S, et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186(10):5648–55. doi: 10.4049/jimmunol.1003161
- Haymaker C, Wu R, Bernatchez C, et al. PD-1 and BTLA and CD8(+) T-cell “exhaustion” in cancer: “Exercising” an alternative viewpoint. Oncoimmunology. 2012;1(5):735–8. doi: 10.4161/onci.20823
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
- Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol. 2010;22(1):7–12. doi: 10.1093/intimm/dxp112
- Eto D, Lao C, DiToro D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4T cell (Tfh) differentiation. PloS One. 2011;6(3):e17739–00. doi: 10.1371/journal.pone.0017739
- Pelletier N, McHeyzer-Williams LJ, Wong KA, et al. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol. 2010;11(12):1110–8. doi: 10.1038/ni.1954
- Cui Y, Guan Y, Liu W, et al. [The changes of circulating follicular regulatory T cells and follicular T helper cells in children immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi. 2014;35(11):980–4.
- Zhang Q, Bai H, Wang W. Increased percentages of T cells producing interleukin-21 in patients with immune thrombocytopenic purpura. Cell Biol Int. 2014;38(4):520–5. doi: 10.1002/cbin.10220
- Grogg KL, Attygalle AD, Macon WR, et al. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod Pathol. 2006;19(8):1101–7.
- Battistella M, Beylot-Barry M, Bachelez H, et al. Primary cutaneous follicular helper T-cell lymphoma: a new subtype of cutaneous T-cell lymphoma reported in a series of 5 cases. Arch Dermatol. 2012;148(7):832–9. doi: 10.1001/archdermatol.2011.3269
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–83. doi: 10.1016/S1470-2045(16)30053-5
