ABSTRACT
Objective: This study aims to explore the clinical features of multiple myeloma (MM) and the influence of various prognostic factors on survival.
Methods: A retrospective analysis, consisting of clinical characteristics analysis and laboratory examinations, was performed on 787 MM patients. Clinical and laboratory parameters were analyzed by multivariate process and compared across different groups.
Results: Of the 787 patients enrolled (median age, 61 years old, range 29–89 years old), 491 (62.4%) were male. Two most common complaints were bone pain (51.2%) and fatigue (48.0%). Anemia (hemoglobin (Hb) ≤100 g/L in female, Hb ≤110 g/L in male) was present initially in 69.4% patients. IgG was the most common type (46.6%). 52.2% of the patients were diagnosed on stage IIIA according to Durie–Salmon (D–S) system, 44.6% are on stage III according to International Staging System (ISS). Multivariate analysis suggested that age, serum calcium, LDH, percentage of abnormal plasma cells in bone marrow were all independent prognostic factors for OS.
Conclusion: The MM patients in China are relatively younger, have higher rate on stage III according to D–S system. Older age, high serum calcium, high LDH, high percentage of abnormal plasma cells in bone marrow were highly related to poor prognosis.
Multiple myeloma (MM) is a B-cell malignancy, morphologically characterized by a monoclonal proliferation of plasma cells in the bone marrow that impairs hematopoiesis, activates osteoclastic bone resorption, and secretes a monoclonal paraprotein (M-protein) in serum and/or urine [Citation1]. MM accounts for about 1% of human neoplasms, almost 2% of deaths due to cancers, and 12–15% of all cases of hematological malignancy [Citation2,Citation3].
The etiology of MM is unknown. Epidemiologic data suggests that age, genetic factors, chronic antigenic stimulation, and some environmental or occupational factors may play a role in pathogenesis of MM [Gahrton, 2004 #1223]. Intriguingly, the variation in worldwide incidence is much greater for MM than for other hematologic malignancies [Citation4]. The reported age-adjusted incidence per 100,000 population of MM around the world is 0.5 in Hawaiian Japanese, 0.9–3.3 in most European countries, and 8.2 in San Francisco Bay Area black men [Citation5–7].
Materials and methods
Patients
This is a single-center hospital-based retrospective descriptive study conducted on 787 confirmed new-diagnosed multiple myeloma (MM) cases from January 2006 to June 2014. All the diagnoses were performed according to the standard [Citation8] of International Myeloma Working Group (IMWG) of MM. All patients’ laboratory reports were reviewed and verified by our staff. Patients, with plasma cell leukemia (PCL), monoclonal gammopathy of undetermined significance, systemic amyloidosis, or other conditions associated with a monoclonal gammopathy as defined previously [Citation9], were excluded.
Methods
Recorded materials
The age of onset, sex of patients, chief complains, disease type, complications, D–S stage, ISS stage, and outcomes of tests including Hb, percentage of abnormal plasma cells, Scr, albumin, serum calcium, β2-MG, LDH, CRP and FISH characteristics of all patients were recorded and analyzed.
(2) Follow-ups
Till December 2014, all the patients were monitored with calling to patients or their families and properly recorded.
(3) Statistics
All analyses were performed using SPSS 19.0 software (SPSS Institute). Kaplan–Meier survival curves were used for estimation of OS. Censoring for those alive at last follow up. Log-rank test used for significance test, and Cox regression model for multivariate analysis. All directional P values were 2-tailed, with a P 0.05 considered significant for all tests.
Results
Incidence
From January 2006 to June 2014, a total of 787 newly diagnosed MM patients including 491 (62.4%) men and 296 (37.6%) women were registered in our hospital, the ratio between male and female was around 2:1 (1.6:1).
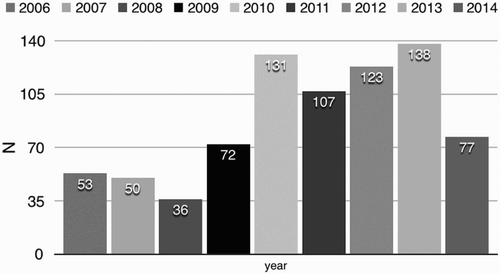
The numbers of new-diagnosed MM patients have increased on a yearly basis, the detailed data are shown in .
Clinical characteristics
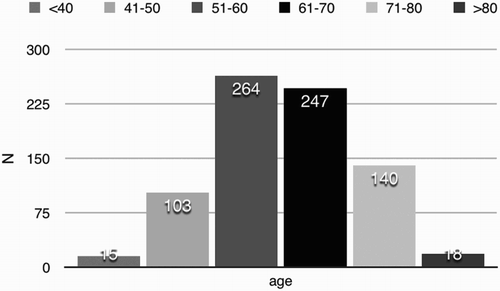
The median of ‘age of onset’ was 61 years old (ranging from 29 to 89 years old). The peak range was 51–60 years, with a total number of 264 (33.5%); the second range was 61–70 years, with a total number of 247(31.4%). The detail data are listed in .
Among the 787 MM patients, the most common signs were bone pain and fatigue, the percentage was 52.1 and 48.0%, respectively. Following with these two signs, the third common manifestation was abnormal urea, including but not limited to foam urea, oliguresis, edema and increased night urine. 67 in 787 patients (8.5%) complained fever as diagnosed. Some patients’ chief complains were bleeding, mass, suppression in the chest and so on, but all these mentioned above were seldom seen.
The most common accompanying diseases were hypertension (267 in 787, 34.0%), and chronic hepatitis (66 in 787, 8.4%), and diabetes (60 in 787, 7.6%).
Characteristics of laboratory results
The mean values and ranges of characteristics of laboratory results were listed in . Among these patients, severe anemia patients (<60 g/L) were 92 (11.7%), patients with ratio of plasma cells in bone marrow over 30% were 404 (51.3%). Patients with abnormal renal function (Scr ≥ 176.8 µmol/L) were 194 (24.7%), 381 (48.4%) with hypoproteinemia (<35.0 g/L), 86 (10.9%) with hypercalcemia (≥2.75 mmol/L), 224 (63.7%) with CRP above high normal level (>8.2 mg/L), 161 (26.7%) with LDH above high normal level (≥240U/L).
Table 1. The mean value and range of laboratory outcomes.
Mentioned β2-MG, the number (β2-MG < 3.5 mg/L) was 258 in 787 (32.8%), 328 in 787 (41.7%) with β2-MG ≥ 5.5 mg/L, and 201 in 787 (25.5%) with β2-MG range from 3.5 to 5.5 mg/L.
Among 175 patients who received fluorescence in situ hybridization (FISH) tests 119 (68%) had abnormal chromosome. The most common abnormality was 1q21 amplification, 66 in 119 (37.7%). The IgH rearrange, Rb1 deletion, D13S319 deletion were 55 (31.4%), 51 (31.4%), 34 (19.4%), respectively. Twenty-five out of 119 had p53 deletion, occupied 14.3%. Among these 119 patients, patients who had ≥3 types abnormalities of chromosome was 31 in 119 (26.0%).
Type and stage
The detailed data of the categories of 787 MM patients have been listed in . The most common type was IgG, 366 out of 787 (46.5%). Ranking second was IgA, 203 out of 787 (26.4%), then light-chain type, 188 out of 787 (23.1%).
Table 2. The type of 787 MM patients.
The details of the stage of these MM patients (according to D–S and ISS) were listed in . Of the D–S stage, the total number of MM patients with renal function abnormality was 194 (24.7%) in 787.
Table 3. The stage of 787 MM patients according to D–S and ISS.
Overall survival
Among the 787 patients, the loss of follow up rate was 26.18% (206 out of 787). By the end of the monitoring process, 332 MM patients survived. The first year survival rate was 85.8%, third year survival rate was 64.0%, and fifth year survival rate was 42.5%. In the end, 548 patients remained with over 6 months completed following up data. The relationship between clinical characteristics and survival can be seen in . Multivariate analysis suggested that age (HR 1.675, P = 0.002), serum calcium (HR 1.679, P = 0.032), LDH (HR 1.797, P = 0.001), percentage of abnormal plasma cells in bone marrow (HR 1.501, P = 0.023) were all independent prognostic factors for OS.
Table 4. The relationship between clinical characteristics and overall survival.
Discussion
Multiple myeloma is the malignant counterpart of long-lived plasma cells with a strong tropism for bone and bone marrow. The median age multiple myeloma being diagnosis is 69 years, with three-quarters of patients being diagnosed at the age of over 55 years and 2/3 of the patients being men [Citation8]. To our best knowledge, there are few reports to comprehensively describe the epidemiology of MM in Chinese. To address the incidence, clinical features and prognosis of MM, data of 787 MM patients were analyzed as sample. Our data suggests that, out of the 787 patients, 491 (62.4%) were man. Median age of the whole group was 61 years old (ranging from 29 to 89 years old). In Asian countries, there is growing evidence that recognition of MM is increasing rapidly, doubled MM incidence in the last 10 years [Citation9–11]. In our study, the incidence increased more consistently since 2010 than that of other time period, which suggested a dominant period effect. In addition to age effect, both period and cohort effects contributed to the increasing trend in incidence of MM. Multiple factors, including improvement in diagnostic techniques and case ascertainment [Citation12], may be involved in the period effect.
The most common clinical manifestations of symptomatic multiple myeloma are anemia, infections, lytic or osteopenic bone disease, or renal failure. However, patients with multiple myeloma might be diagnosed at an asymptomatic stage by chance. Generally, multiple myeloma is diagnosed at an earlier stage today than in the past [Citation13]. In our study, the most common complains of these MM patients at diagnosed were bone pain (51.2%) and fatigue (48.0%).
Greipp et al. [Citation14] concluded the types of 10,750 MM patients in their study: the percentage of IgG, IgA, IgD and light-chain type was 60, 24, 3 and 11%, respectively. In our study, the most common type was IgG type, 366 out of 787 (46.5%). Following common type was IgA, 203 in 787 (26.4%), then the light-chain type, 188 in 787 (23.1%). It has been found out that there were 584 in 787 (74.2%) patients who were in stage III according to D–S stage. The atypic clinic manifestation and latent upset might be the reason for this. Back pain, particularly in older patients, or unreason anemia should prompt screening for the presence of multiple myeloma, so that the disease may be diagnosed and treated as early as possible.
In recent years, application of new drugs improves the prognosis of MM patients, including those of the elderly [Citation15]. In our study, the loss of follow up rate in 787 patients was 26.18% (206 in 787). By the end of following up, 332 MM patients survived. The first year survival rate was 85.8%, third year survival rate was 64.0%, and fifth year survival rate was 42.5%. In the end, 548 patients remained with over 6 months completed following up data. The relationship between clinical characteristics and survival can be seen in . Multivariate analysis suggested that age, Hb, albumin, serum calcium, β2-MG, LDH, CRP, ratio of plasma cells and percentage of abnormal plasma cells in bone marrow were all independent prognostic factors for OS. Here, we reviewed patient and disease characteristics reported in key interventional and observational studies in MM (including sex, age, hemoglobin, creatinine, albumin, calcium, β2-MG, LDH, CRP, ratio of plasma cells, percentage of abnormal plasma cells in bone marrow, therapy, D–S stage and ISS stage) to examine common and disparate features of patients with MM.
Age is a significant prognostic factor for patients with MM. Patients who are >50 years of age at diagnosis displayed significantly shorter median survival times than younger patients [Citation16]. In this study, patients who >60 years of age at diagnosis displayed significantly shorter median survival times than younger patients. The older patients have frailty which induced poor chemotherapy endurance in common. While frailty has been found to be a significant prognostic factor for patients with MM [Citation17], this study also shows that the hemoglobin is an independent prognostic factors for OS, the level of hemoglobin being less than 100 g/L suggests poor prognosis. Renal function is an important characteristic for patients with MM, as the development of renal failure is a negative prognostic factor for patient survival [Citation18–20]. Renal dysfunction is a common comorbidity in patients with MM. This study shows that if renal function is less than 176.8 mmol/L then ir is a sign of good prognosis.
Apart from the information mentioned above, other high-risk indicators of MM include tumor burden and staging [Citation21]. β2-MG is a serum marker of tumor burden in patients with MM and has been shown to be prognostic for patient survival [Citation22–24]. Two hundred and fourteen MM patients out of 548 have high levels of β2-MG (≥5.5 mg/L) and shorter OS. Ratio of plasma cell and percentage of abnormal plasma cells in bone marrow are also related to disease burden. If the ratio of plasma cells ≥30% and the percentage of abnormal plasma cells in bone marrow >0.8, it demonstrates poor prognosis as well. Serum β2-MG and albumin are the basis of ISS [Citation25]. International Staging System based on serum albumin and β2-microglobulin levels, is the most important clinical prognostic model predicting survival in myeloma [Citation14]. ISS stage is another indicator of OS, and this study shows that MM patients with poor survival determined by ISS staging (e.g. ISS stage II or III). The hemoglobin, calcium and renal dysfunction are closely related D–S, thus D–S stage is also a key indicator of OS in this study.
Elevated LDH has been found to be an adverse prognostic indicator for survival in patients with MM [Citation26,Citation27]. Our study proves that if the level of LDH of MM patients is ≥240U/L, poor survival is a highly possible result. We also find that the level of CRP (≥8.2 mg/L) and calcium (≥2.75 mmol/L) is associated with shorter survival, which has not been discovered in the previous study.
Most importantly, a number of patient- and disease-related factors can also have an effect on treatment choice, treatment efficacy, and tolerability; thus, an understanding of the heterogeneity of patients in the setting of MM is important for appropriate treatment selection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mrs. Jiejing Qian is a faculty member at hematology department of 1st affiliated hospital of Zhejiang university, Zhejiang, China. She is an expert in hematological malignancies.
Dr. Jie Jin is a leader at hematology department of 1st affiliated hospital of Zhejiang university, Zhejiang, China. She is a professor in hematological malignancies.
Mr. Hong Luo is a faculty member at hematology department of 1st hospital of Qiqihar, Heilongjiang, China. He is a researcher of epidemiology.
Mrs. Chunji Jin is a faculty member at hematology department of 1st affiliated hospital of Zhejiang university, Zhejiang, China. She is a resident doctor.
Mrs. Lei Wang is a faculty member at hematology department of 1st affiliated hospital of Zhejiang university, Zhejiang, China. She is an expert in the field of statistics.
Dr. Wenbin Qian is a chief physician at hematology department of 1st affiliated hospital of Zhejiang university, Zhejiang, China. He is an expert in lymphoma and statistics.
Dr. Haitao Meng is a chief physician at hematology department of 1st affiliated hospital of Zhejiang university, Zhejiang, China. He is an expert in multiple myeloma and epidemiology.
Additional information
Funding
References
- Gahrton G, Durie B, Samson D. Multiple myeloma and related disorders. Lancet Oncol. 2004;5(12):765.
- Devesa SS. Descriptive epidemiology of multiple myeloma. Berlin, Heidelberg: Springer; 1991.
- Greenlee MRT, Murray MT, Bolden MS, et al. Cancer statistics. Cancer J Clin. 2000;50(1):733. doi: 10.3322/canjclin.50.1.7
- Vineis P. Incidence and time trends for lymphomas, leukemias and myelomas: hypothesis generation. Leukemia Res. 1996;20(4):285–290. doi: 10.1016/0145-2126(95)00153-0
- Phekoo KJ, Schey SA, Richards MA, et al. A population study to define the incidence and survival of multiple myeloma in a national health service region in UK. Br J Haematol. 2004;127(3):299–304. doi: 10.1111/j.1365-2141.2004.05207.x
- Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225–247.
- Cartwright RA, Gilman EA, Gurney KA. Time trends in incidence of haematological malignancies and related conditions. Br J Haematol. 1999;106(2):281–295. doi: 10.1046/j.1365-2141.1999.01480.x
- National Cancer Institute. Surveillance epidemiology and end results program. SEER stat fact sheets on multiple myeloma; 2013 [cited 2013 Apr 24]. Available from: http://seer.cancer.gov/statfacts/html/mulmy.html
- Kim K, Lee JH, Kim JS, et al. Clinical profiles of multiple myeloma in Asia – an Asian myeloma network study. Am J Hematol. 2014;89(7):751–756. doi: 10.1002/ajh.23731
- Lee JH, Lee DS, Lee JJ, et al. Multiple myeloma in Korea: past, present, and future perspectives. Experience of the Korean multiple myeloma working party. Int J Hematol. 2010;92(1):52–57. doi: 10.1007/s12185-010-0617-6
- Huang SY, Yao M, Tang JL, et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer. 2007;110(4):896–905. doi: 10.1002/cncr.22850
- McKean-Cowdin R, Feigelson HS, Ross RK, et al. Declining cancer rates in the 1990s. J Clin Oncol. 2000;18(11):2258–2268. doi: 10.1200/JCO.2000.18.11.2258
- Riccardi A, Gobbi PG, Ucci G, et al. Changing clinical presentation of multiple myeloma. Eur J Cancer. 1991;27(11):1401–1405. doi: 10.1016/0277-5379(91)90020-E
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313
- Ludwig H, Durie BV, Turesson I, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–4047. doi: 10.1182/blood-2007-03-081018
- Antonio P, Sara B, Maria-Victoria M, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an international myeloma working group report. Blood. 2015;125(13):2068–2074. doi: 10.1182/blood-2014-12-615187
- Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65(3):175–181. doi: 10.1034/j.1600-0609.2000.90221.x
- Kleber M, Ihorst G, Deschler B, et al. Detection of renal impairment as one specific comorbidity factor in multiple myeloma: multicenter study in 198 consecutive patients. European J Haematol. 2009;83(6):519–527. doi: 10.1111/j.1600-0609.2009.01318.x
- Augustson BM, Gulnaz B, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom medical research council trials between 1980 and 2002 – Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219–9226. doi: 10.1200/JCO.2005.03.2086
- Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28(2):269–277. doi: 10.1038/leu.2013.247
- Bataille R, Grenier J, Sany J. Beta-2-microglobulin in myeloma: optimal use for staging, prognosis, and treatment – a prospective study of 160 patients. Blood. 1984;63(2):468–476.
- Durie BG, Stock-Novack D, Salmon SE, et al. Prognostic value of pretreatment serum beta 2 microglobulin in myeloma: a southwest oncology group study. Blood. 1990;75(4):823–830.
- Cuzick J, De Stavola BL, Cooper EH, et al. Long-term prognostic value of serum beta 2 microglobulin in myelomatosis. Br J Haematol. 1990;75(4):506–510. doi: 10.1111/j.1365-2141.1990.tb07790.x
- Warren JL, Harlan LC, Stevens J, et al. Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol. 2013;31(16):1984–1989. doi: 10.1200/JCO.2012.46.3323
- Kiba T, Ito T, Nakashima T, et al. Bortezomib and dexamethasone for multiple myeloma: higher AST and LDH levels associated with a worse prognosis on overall survival. BMC Cancer. 2014;14:78. doi: 10.1186/1471-2407-14-462
- Gkotzamanidou M, Kastritis E, Nikitas N, et al. Increased serum lactate dehydrogenase should be included among the variables that define very-high-risk multiple myeloma. Clin Lymph Myelom Leuk. 2011;11(11):409–413. doi: 10.1016/j.clml.2011.07.001