ABSTRACT
Objectives: Encouraging progress has been made in application of splenectomy in the treatment of relapsed hemophagocytic lymphohistiocytosis (HLH) of unknown cause. The aim was to determine the roles of lymphocyte subpopulations and inflammatory cytokines in splenectomy.
Methods: We retrospectively analyzed changes in lymphocyte subpopulations and levels of inflammatory cytokines at different time-points before and after splenectomy in the patients with relapsed HLH of unknown cause, as well as the correlations between these changes and the disease prognosis.
Results: During the period from June 2006 to June 2016, we enrolled 107 patients with relapsed HLH of unknown cause, of whom 29 were treated with splenectomy. Among the 29 patients, 7 cases were non-Hodgkin lymphomas based on spleen pathology, 1 case withdrew and the remaining 21 non-lymphoma cases were available for analysis. Results showed a significant increase in both percentage of CD16+CD56+ NK cells (P = 0.003) and NK cell activity (P = 0.028) at 24 wk after splenectomy compared to their baseline pre-surgery levels. We also examined seven patients for the changes in cytokine levels before and after splenectomy and found that IL-21 and IL-1α decreased at 4 wk after splenectomy (P < 0.05). Seven non-lymphoma patients determined as no response to treatment (NR) prior to splenectomy had significantly longer survival (P = 0.001) compared to the 24 patients with relapsed HLH of unknown cause who were also determined as NR but not treated by splenectomy.
Discussion: Splenectomy can improve clinical symptoms and survival of patients with relapsed HLH of unknown cause. The mechanism is likely related to the changes in percent NK cells and cytokines (IL-21 and IL-1α) after surgery.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is an immune disorder characterized by uncontrolled activation of T cells and macrophages and excessive production of inflammatory cytokines. HLH is classified as either primary or secondary disease based on the nature of cause. For some HLH patients, the cause can be attributed to the underlying diseases, for others however, no clear reason can be found, thus these patients are diagnosed as ‘HLH of unknown cause’. The patients in the latter category usually comprise cases of refractory/relapsed HLH. Several studies have reported therapeutic effect of splenectomy on HLH [Citation1,Citation2]. A retrospective analysis of clinical data collected from 19 patients with relapsed adult HLH of unknown cause treated with splenectomy showed promising results as the follow-up on 18 patients for a median of 25 mo (3–79 mo) showed that 11 cases survived, with 48 and 24% progression-free survival (PFS), and 57 and 25% overall survival (OS), at 12 and 36 mo, respectively; the median survival time was 22 mo, and a 16-y old male patient had a symptom-free survival up to 57 mo [Citation3]. Since spleen contains a large population of important immune cells and immune-active molecules, we hypothesized that splenectomy may exert its therapeutic effect on HLH through regulating inflammation and production of anti-inflammatory molecules. Herein, we determined changes in lymphocyte subpopulations and levels of inflammatory cytokines at different time-points, as well as the correlations between these changes and disease prognosis. The findings of this study will help clinicians understand the mechanisms underlying the therapeutic effect of splenectomy in treating HLH of unknown cause.
Methods
Diagnosis criteria
HLH diagnosis was made according to the internationally accepted guidelines HLH-2004 diagnostic criteria [Citation4]. This study was approved by the Ethics Committee of Hospital. Patients provided consent to be treated with diagnostic/therapeutic splenectomy. The clinical and laboratory data of patients were analyzed retrospectively.
Screening for the cause of HLH
The following examinations were conducted to determine the cause of HLH: (1) high-throughput HLH-associated gene sequencing; (2) parameters related to rheumatic immune diseases, and also further biopsies of skin and labial gland if needed; (3) pathogen tests for bacteria, fungi, tuberculosis, virus, and atypical pathogens in blood, urine, stool, and tissue samples; and (4) cancer-related tests including tumor markers, positron emission technology-computer tomography (PET-CT), biopsies of bone marrow, lymph nodes, and suspicious lesion tissues. These tests were performed to rule out the HLH caused by other diseases such as rheumatic diseases, infections, and cancer (in particular, lymphomas).
Observation parameters
Lymphocyte subpopulations were examined at first visit, 1 wk prior to splenectomy, and 2 and 24 wk after splenectomy. Serum concentrations of inflammatory cytokines interferon-gamma (IFN-γ), IL-6, tumor necrosis factor-alpha (TNF-α), IL-21, and IL-1α at 1 wk prior to, and 4 wk after splenectomy were analyzed using Luminex method (eBioscience, Cat#EPX450-12171-901).
Criteria for therapy assessment
Therapeutic efficacy was assessed according to the criteria proposed by Marsh et al. [Citation5]: Complete response (CR) was defined as absence of clinical symptoms, and normalization of all the HLH-related laboratory tests. Partial response (PR) was defined as at least a 25% improvement in two or more symptoms and laboratory tests including sCD25, ferritin, and triglyceride; for the patients with initial absolute neutrophil count (ANC) <0.5 × 109/l, the values should be elevated to >0.5 × 109/l; for those with an initial ANC at 0.5–2.0 × 109/l, the values should be elevated to >5.0 × 109/l; for the patients with ALT >400 U/l, the values should be decreased by 50%; absence of hemophagocytosis; consciousness recovered to normal in patients with refractory central nervous system HLH and reduced levels of consciousness. No response (NR) was defined as failing to achieve PR criteria.
Survival
Survival time was calculated from the diagnosis as relapsed HLH to death or 1 December 2016 in the follow-up.
Statistical analysis
Data was analyzed using SPSS 16.0 software. Due to the small sample size, data with non-normal distribution were presented as medians and ranges, and multi-sample comparisons were conducted using Wilcoxon rank-sum test. The differences at P values <0.05 were considered statistically. Survival data were analyzed using Kaplan–Meier survival plots and the log-rank test was used to compare the survival times between groups.
Results
General characteristics of the patients
From June 2006 to June 2016, 503 patients with HLH were enrolled in the Department of Hematology, Beijing Friendship Hospital, Capital Medical University. Primary diseases included: 18 cases of primary HLH; 184 cases of infection associated HLH; 126 cases of malignant tumor associated HLH; 49 cases of rheumatic disease associated HLH; 13 cases of other causes; 113 cases of unknown cause, of whom 107 cases of recurrence. 107 patients with relapsed HLH of unknown cause included 53 males, 54 females, with a median age at 36 y (range 3–84 y), of whom 29 received splenectomy treatment. The 29 patients who were treated with splenectomy were almost equally distributed between sex (15 males, 14 females), and had a median age of disease onset at 37 y (range 7–63 y), with 24 of them (82.8%) being 18 y or older. The clinical characteristics of these patients are shown in .
Table 1. Clinical characteristics of 29 patients with relapsed HLH of unknown cause.
Changes in lymphocyte subpopulations and NK cell activity in response to splenectomy
Among the 29 patients, 7 cases were diagnosed as non-Hodgkin lymphomas based on spleen pathology. In the remaining 22 non-lymphoma patients, one subject withdrew from the study thus 21 cases were available for analysis. shows the percent CD3+ cells (T cells), CD4+ T cells, CD8+ T cells, CD4+/CD8+ T cell ratio, CD19+ cells (B cells), and CD16+CD56+ cells (NK cells) measured at the first visit, before splenectomy, and 2 and 24 wk after splenectomy in the 21 patients. Results showed a significantly higher percentage of NK cells (P = 0.003) and NK cell activity (P = 0.028) at 24 wk after treatment compared to before treatment, while no significant changes were found for all the other cell populations examined ().
Table 2. Changes in lymphocyte subpopulations and NK cell activity at different time-points before and after splenectomy.
Changes in cytokine expression in response to splenectomy
Of 29 patients treated with splenectomy, 7 patients were examined for their serum levels of cytokines. Results showed that IL-21 and IL-1α were decreased (P < 0.05) at 4 wk after splenectomy, whereas IFN-γ, IL-6, TNF-α were not significantly changed compared to pre-surgery ().
Table 3. Changes in cytokines before and after splenectomy in 7 HLH patients.
Correlations between lymphocyte subpopulations, cytokines, and therapeutic effect of splenectomy
After splenectomy, 21 non-lymphoma patients continued to receive treatment for 2–3 mo. There were eight cases of CR, nine cases of PR, and four cases of NR after splenectomy. There was no difference between CR + PR and NR patients in the pre-treatment time-point in the percentage of CD3+T cells, CD4+ T cells, CD8+ T cells, CD4+/CD8+ T cell ratio, CD19+B cells, and CD16+CD56+ NK cells. In the seven patients whose serum cytokines were examined, there was no significant correlation between pre-treatment cytokine levels and therapeutic effect; however, we found that IFN-γ levels was reduced in PR + CR patients, but elevated in NR patients at 4 wk after splenectomy than before splenectomy ().
Table 4. IFN-γ levels and treatment effectiveness before and after splenectomy in seven HLH patients.
Survival time
In the 21 non-lymphoma patients before splenectomy, there were 14 of PR and 7 cases of NR. Up to 1 December of 2016, there were 13 cases of survival and 8 cases of death, and total mortality rate was 38.1% (8/21 cases). Of the eight fatalities, five patients died from primary diseases, two patients died from lung infection, and one patient who had HLH relapse after splenectomy was treated with nonidentical allogeneic hematopoietic stem cell transplantation and died from transplant complication.
Among 78 patients with relapsed HLH of unknown cause who were not treated with splenectomy, 5 lost contact with the study site; and in the remaining 73 patients, there were 7 cases of CR, 42 cases of PR, and 24 cases of NR. There were 38 cases of survival, 35 cases of death, and total mortality rate was 47.9% (35/73 cases).
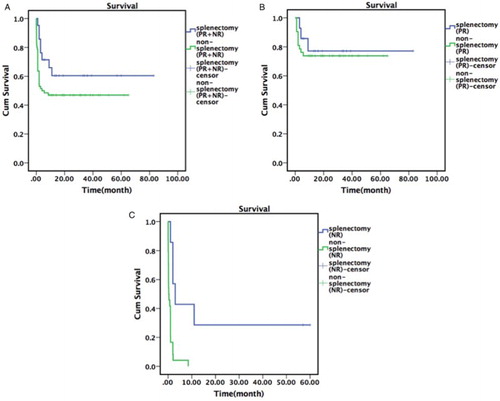
There was an insignificant increase (P = 0.111) in survival time in the 21 non-lymphoma patients with pre-splenectomy PR + NR compared to the 66 patients with PR + NR but not treated with splenectomy. There was no difference in survival time between the 14 non-lymphoma patients with pre-splenectomy PR and the 42 patients with PR but not treated with splenectomy (P = 0.654). There was a significant increase (P = 0.001) in survival time in the 7 non-lymphoma patients with pre-splenectomy NR compared to the 24 patients with NR but not treated with splenectomy ().
Figure 1. Survival time of patients with relapsed HLH of unknown cause. (a) Comparison of total survival between the patients (PR + NR) with relapsed HLH of unknown cause who were treated with splenectomy vs. or those not treated with splenectomy. (b) Comparison of total survival between the patients (PR) with relapsed HLH of unknown cause who were treated with splenectomy vs. those not treated with splenectomy. (c) Comparison of total survival between the patients (NR) with relapsed HLH of unknown cause who were treated with splenectomy vs. those not treated with splenectomy.
Discussion
HLH is a clinical syndrome associated with hereditary or acquired immune-dysregulation. HLH pathology is mainly characterized by abnormal activation and proliferation of lymphocytes, monocytes, and macrophages, as well as excessive production of cytokines by these cells, leading to hyperactive inflammatory response [Citation6]. HLH clinical manifestations include fever, hepatosplenomegaly, pancytopenia, and hemophagocytosis in bone marrow, liver, spleen, and lymph nodes. HLH is classified into either primary or secondary disease based on whether or not clear genetic defects are present. Patients of primary HLH have clear hereditary or genetic defects, and the disease often starts in infancy or young age, and shows functional deficiency of cytotoxicity. Patients of secondary HLH have no familial history or known genetic defects, and etiology of the disease is related to a variety of triggering factors such infection, cancer, and rheumatic disease. Our previous study found that in some HLH patients of unknown cause, splenic lymphoma was the primary disease [Citation3]. In the current study, 7 HLH patients were diagnosed with lymphoma according to spleen pathology after splenectomy. Several studies have reported the therapeutic effect of splenectomy on HLH. For example, Zhang et al. [Citation2] reported that after splenectomy, a 56-y old patient with HLH of unknown cause who did not previously respond to CHOP treatment showed improvement. Likewise, Imashuku et al. [Citation1] reported short-term therapeutic effect of splenectomy in five pediatric patients with HLH. Chen et al. [Citation7] summarized splenectomy treatment in eight cases of HLH with unknown cause and suggested that for patients with splenomegaly, the use of splenectomy not only helped with diagnosis, but it also served as a therapeutic method. In the current study, 29 (PR + NR) patients with relapsed HLH of unknown reason received splenectomy. In the 21 non-lymphoma patients accessible for follow-up (pre-splenectomy 14 cases of PR, 7 cases of NR), 8 patients had CR and 9 patients had PR after splenectomy, suggesting that splenectomy may improve the disease condition in the patients who did not achieve CR, and previous studies have shown a correlation between CR and patient long-term survival [Citation3]. Up to 1 December 2016, in these 21 patients, there were 13 cases of survival and 8 cases of death, and total mortality rate was 38.1% (8/21 cases). In comparison, in the 73 patients with relapsed HLH of unknown cause who were not treated with splenectomy, there were 38 cases of survival, 35 cases of death, and total mortality rate was 47.9% (35/73 cases). However, the difference in survival between patients (PR + NR) with relapsed HLH of unknown cause who were treated and not treated with splenectomy was not statistically significant (P = 0.111). Nonetheless, splenectomy significantly increased survival time (P = 0.001) in the patients with pre-splenectomy NR, suggesting that splenectomy may be an ideal treatment strategy when relapsed HLH of unknown cause fails to achieve CR after standard therapy. This method may improve disease condition and extend survival in some patients, particularly for those who have NR.
Pathophysiology of HLH is believed to be multiple factor-induced decrease or loss of in NK cell and cytotoxic T lymphocyte functions leading to ‘cytokine storm’ and consequently, organ failure [Citation8]. The decrease or lack in NK cell activity in HLH patients is a milestone finding and thus NK activity assay has become one of important markers for HLH diagnosis. In both primary and secondary HLH, decreased or absent NK activity has been observed during the development of HLH. In the patients with primary HLH, while the number of NK cells may be normal, their degranulation and cytotoxic activity are diminished [Citation9]. In patients with secondary HLH, both number and cytotoxic activity of NK cells may be reduced during the active phase of disease, and return to normal after treatment [Citation10]. Since spleen is enriched with important immune cells and the immune-active molecules produced by these cells, we hypothesized that the therapeutic effect of splenectomy on HLH may be mediated through altering immune cell functions and production of molecules that regulate inflammation. In the current study, we found that patients with relapsed HLH of unknown cause had increased NK cell activity (P = 0.028), as well as higher percent NK cells in peripheral blood (P = 0.003) at 24 wk after splenectomy compared to before surgery. These results indicate that in active phase of HLH, both the number of NK cells and their cytolytic activity against target cells are decreased and there is a dysregulated immune function. It can be speculated that splenectomy may increase number of NK cells and increase their activity, even back to normal levels, resulting in an improvement of HLH.
According to the current view, HLH development is caused by dysregulated immune responses involving excessive production of cytokines (cytokine storm) by activated lymphocytes and macrophages. In particular, abnormally activated CD4+ T cells produce large amount of cytokines such as TNF-α, IL-6, and IFN-γ. These cytokines can stimulate monocytes/macrophages and promote their rapid activation and proliferation, and this process in turn, results in increased cytokine production. High levels of cytokines can cause fever, overproduction of IFN-γ and TNF-α can induce bone marrow hematopoietic inhibition, and lymphocyte infiltration can cause hepatosplenomegaly and abnormal liver function. Several studies have shown that splenectomy can reduce the number of inflammatory cells (T cells, neutrophils, and macrophages) and production of inflammatory cytokines (IL-1β and TNF-α) [Citation11,Citation12]. In the current study, we found significant reduction of IL-21 and IL-1α (P < 0.05) 4 wk after splenectomy. IL-21 influences differentiation, proliferation, and functions of both T cells and B cells. For example, IL-21 can induce maturation of CD8+ T cells and NK cells and increase their cytotoxicity. IL-21 can also induce differentiation of naïve B cells and memory B cells into plasma cells. In addition, IL-21 impacts the development of different T cell subtypes including helper T cells (Th)17, follicular helper T cells (TFH), regulatory T cells (Tregs), and promotes development of autoimmune and inflammatory diseases, while blockage of IL-21 has been shown to improve autoimmune and inflammatory diseases in animal models [Citation13]. IL-1α is a major pro-inflammatory mediator that can induce fever and immune activation by binding to IL-1 receptor [Citation14], and several studies have shown that IL-1 antagonist anakinra is effective in treating HLH [Citation15,Citation16]. The results of the current study suggest that improved disease condition and prognosis after splenectomy in the patients with relapsed HLH of unknown cause may be related to reduced levels of inflammatory cytokines such as IL-21 and IL-1α.
IFN-γ is believed to be a key cytokine in pathogenesis of HLH. IFN-γ is a pro-inflammatory cytokine produced by NK cells and antigen presenting cell (APC)-activated T cells. A major function of IFN-γ is to activate monocytes and macrophages [Citation17]. IFN-γ plays a key role in development of primary HLH. In this patient population, IFN-γ was found to be significantly increased, and together with dysregulated production of other pro-inflammatory cytokines such as TNF-α and IL-6, the levels of these cytokines can be rapidly resumed to normal after effective treatment of the disease [Citation18,Citation19]. Elevated IFN-γ is also found in several animal models of primary HLH, and its normalization has been shown to substantially increase the survival [Citation20,Citation21]. Application of IFN-γ blocking antibody in HLH therapy is currently underway in clinical trials [Citation22]. In the current study, we found no change in peripheral blood IFN-γ levels after splenectomy. However, we were still able to confirm the relationship between IFN-γ and disease condition in HLH patients since we found that IFN-γ levels in PR + CR patients was reduced after splenectomy, and in particular, IFN-γ levels were decreased by 20-fold in CR patients but elevated in NR patients at 4 wk after splenectomy. These results indicated that IFN-γ is elevated during the active phase of the disease and reduced during the stable phase of the disease; thus the magnitude of reduction may be used to assess effectiveness of treatment and disease prognosis. Nevertheless, these results require further validation in large scale clinical trials in the future.
In summary, the current study has demonstrated that splenectomy as a therapeutic approach may improve the disease condition and extend survival in the patients with relapsed HLH of unknown cause; the mechanism for this effect may be related to the splenectomy-induced improvement in percent NK cells and levels of cytokines (IL-21 and IL-1α). Although the sample size was small, these results provide useful information to support clinical application of splenectomy in the treatment of relapsed HLH of unknown cause. We are currently conducting a multi-center, prospective clinical trial to investigate the therapeutic effect of splenectomy on relapsed HLH of unknown cause (ClinicalTrials.gov Identifier: NCT02862054).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Imashuku S, Obayashi M, Hosoi G, et al. Splenectomy in haemophagocytic lymphohistiocytosis: report of histopathological changes with CD19+ B-cell depletion and therapeutic results. Br J Haematol. 2000;108(3):505–510. doi: 10.1046/j.1365-2141.2000.01904.x
- Zhang LJ, Zhang SJ, Xu J, et al. Splenectomy for an adult patient with refractory secondary hemophagocytic lymphohistiocytosis. Biomed Pharmacother. 2011;65(6):432–435. doi: 10.1016/j.biopha.2011.04.008
- Jing-Shi W, Yi-Ni W, Lin W, et al. Splenectomy as a treatment for adults with relapsed hemophagocytic lymphohistiocytosis of unknown cause. Ann Hematol. 2015;94(5):753–760. doi: 10.1007/s00277-014-2276-9
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039
- Marsh RA, Allen CE, McClain KL, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60(1):101–109. doi: 10.1002/pbc.24188
- Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38(1):20–31. doi: 10.1080/07853890500465189
- Chen M, Wang S, Duan M, Zhuang J. Diagnostic splenectomy in eight adult patients with hemophagocytic lymphohistiocytosis. J Clin Hematol (China). 2013;26:619–622.
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166(2):95–109. doi: 10.1007/s00431-006-0258-1
- Spessott WA, Sanmillan ML, McCormick ME, et al. Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood. 2015;125(10):1566–1577. doi: 10.1182/blood-2014-11-610816
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X
- Zhang BJ, Men XJ, Lu ZQ, et al. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl). 2013;126(12):2354–2360.
- Shih-Ching K, Choudhry MA, Matsutani T, et al. Splenectomy differentially influences immune responses in various tissue compartments of the body. Cytokine. 2004;28(3):101–108. doi: 10.1016/j.cyto.2004.07.005
- Gharibi T, Majidi J, Kazemi T, et al. Biological effects of IL-21 on different immune cells and its role in autoimmune diseases. Immunobiology. 2016;221(2):357–367. doi: 10.1016/j.imbio.2015.09.021
- Yazdi AS, Ghoreschi K. The interleukin-1 family. Adv Exp Med Biol. 2016;941:21–29. doi: 10.1007/978-94-024-0921-5_2
- Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401–408. doi: 10.1097/PCC.0000000000000078
- Divithotawela C, Garrett P, Westall G, et al. Successful treatment of cytomegalovirus associated hemophagocytic lymphohistiocytosis with the interleukin 1 inhibitor – anakinra. Respirol Case Rep. 2015;4(1):4–6.
- Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252
- Henter JI, Elinder G, Söder O, et al. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922.
- Osugi Y, Hara J, Tagawa S, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89(11):4100–4103.
- Jordan MB, Hildeman D, Kappler J, et al. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–743. doi: 10.1182/blood-2003-10-3413
- Pachlopnik Schmid J, Ho CH, Diana J, et al. A Griscelli syndrome type 2 murine model of hemophagocytic lymphohistiocytosis (HLH). Eur J Immunol. 2008;38(11):3219–3225. doi: 10.1002/eji.200838488
- de Saint Basile G, Ménasché G, Latour S. Inherited defects causing hemophagocytic lymphohistiocytic syndrome. Ann N Y Acad Sci. 2011;1246:64–76. doi: 10.1111/j.1749-6632.2011.06307.x
