ABSTRACT
Background: This study evaluates the efficacy of combined chemotherapy for the management of acute promyelocytic leukemia (APL).
Method: Literature search was carried out in several electronic databases. Meta-analyses were performed to achieve weighted effect sizes of overall survival (OS), disease-free survival (DFS), complete remission (CR) rate, and relapse rate. Metaregression analyses were performed to evaluate the factors affecting CR and relapse rates.
Results: Data from 37 studies (7566 patients) were used for meta-analysis. Median follow-up was 49.24 [95% confidence interval (CI): 41.33, 57.16] months. Five-year OS and DFS were 86.41 [83.97, 88.85] % and 75.42 [67.44, 83.40] %, respectively (pooled effect size [95% CI]). Following induction therapy, 89.77 [87.04, 92.50] % patients achieved CR in 38.25[37.84, 38.65] days and 6.34 [5.98, 6.70] % of the patients died during induction. Induction with all-trans retinoic acid (ATRA), arsenic trioxide (ATO), and daunorubicin (DNR) combination was associated with the highest rate of CR (96.16 [89.92, 92.40] %), followed by ATRA-DNR (94.29 [93.15, 95.43] %), ATRA-DNR-cytarabine (92.04 [88.38, 95.71] %), and ATRA-idarubicin (91.16 [89.92, 92.40] %). Overall relapse rate in the study population was 14.42 [11.97, 16.86] %. Baseline leukocyte count was inversely related to the CR rate.
Conclusion: Combined chemotherapy for APL is associated with 90% CR, 14.4% relapse rate, 86% 5-year OS, and 75% 5-year DFS. Induction with ATRA-DNR-ATO is found better than other combinations with respect to CR and relapse rates. Initial leukocyte count may affect prognosis.
Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia caused by a chimeric protein production that prevents the maturation of myeloid cells at promyelocytic stage [Citation1]. This mutation is due to a balanced reciprocal translocation between chromosomes 15 and 17, resulting in promyelocytic leukemia/retinoic acid receptor alpha (PML/RARA) fusion gene [Citation2,Citation3] which leads to a potentially devastating coagulopathy associated with disseminated intravascular coagulation and primary fibrinolysis. However, this mutation also sets a unique sensitivity to all-trans retinoic acid (ATRA) [Citation4]. Hemorrhagic tendency in APL is characterized by increased procoagulant, fibrinolytic, and proteolytic activities along with thrombocytopenia and thrombosis [Citation5]. Pathogenesis and natural history of APL were well reviewed previously [Citation6–8].
Of all acute non-lymphoblastic leukemias, the incidence of newly diagnosed APL is estimated at 8–15%, representing an incidence of 2–3 cases per million individuals every year. Frequency of APL among myeloid leukemia subtypes seems to be higher in some European populations, in Hispanics of United States, and in some countries of Latin America [Citation9,Citation10]. APL predominantly affects middle-aged adults and has relatively lower incidence in children and elderly [Citation11]. A previous review has revealed that the incidence of APL among all amyloid leukemia cases in children is 5–10% [Citation12]. Benzene exposure is identified as a risk factor and there is also some evidence that implicates increased BMI with the incidence of APL in children [Citation12].
Historically, 6-mercatopurine (6-MP) was used to cure APL at a fast rate, but the prognosis was poor with a remission rate of 5–15%, and the prophylactic therapy that was used to avoid early hemorrhagic death increased the remission rate [Citation13,Citation14]. Later, anthracyclines were trialed. Daunorubicin (DNR) was found to be associated with better remission (13–58%), relapse, and mortality rates, especially at higher doses [Citation15,Citation16]. In mid-1980s, ATRA was introduced for the treatment of APL which, as the sole agent, led to a remission rate of 78–85% [Citation17,Citation18]. As reviewed recently [Citation19], the combinational use of ATRA, cytarabine (AraC), arsenic trioxide (ATO), and anthracyclines, such as DNR and idarubicin (IDA), improved the results by increasing complete remission (CR) to over 90%.
At present, ATRA combined with or followed by anthracycline-based chemotherapy is the reference treatment for newly diagnosed APL which is associated with a CR rate of 85–95% and a 5-year overall survival (OS) rate of 65–70% [Citation11]. Consolidation and maintenance treatments with combining agents, such as 6MP and methotrexate (MTX), to ATRA or AraC may further reduce the relapse risk by 10–15% [Citation20]. Moreover, other drugs are also used for APL in various studies either for induction or consolidation and maintenance. The aim of the present study was to carry out a systematic review of the literature and to perform a meta-analysis of the data generated in relevant studies in order to seek up-to-date evidence regarding the relative efficacies of various combinations of drugs commonly used for the management of APL.
Methods
This study was performed by following Cochrane Collaboration guidelines [Citation21] and is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation22].
Literature search strategy
Literature search was carried out in multiple electronic databases including EMBASE, Google Scholar, OVID SP, PubMed, and Web of Science. Medical subject headings (MeSH) and keywords were used in different combinations. For the primary search, [APL combined therapy] combination was used. For the secondary search, various combinations including [APL all trans retinoic acid (ATRA)], [APL anthracycline/DNR/IDA], [APL cytarabine (AraC)], [APL ATO], [APL chemotherapy], [APL induction], [APL consolidation], [APL maintenance] [APL survival], [APL remission], and [APL relapse] were used. For the tertiary search, various combinations were used from secondary search combinations. The same strategy was used for each database. The search encompassed original research papers published before March 2015 in English language. Bibliographies of important relevant research articles were manually searched.
Inclusion and exclusion criteria
The inclusion criteria were: (a) randomized controlled/clinical trial (RCT), prospective or retrospective study recruiting APL patients after the confirmation of pathognomonic PML/RARA receptor gene rearrangement by cytogenetic, fluorescence in situ hybridization, polymerase chain reaction or related molecular tests; (b) the study evaluated the efficacy of a combined chemotherapeutic regimen for the treatment of APL in newly diagnosed patients; (c) the study followed the patients for at least one year; and (d) the study reported CR, relapse, and survival rates. The study was excluded if it recruited relapsed cases; utilized autologous transplantation; reported only tolerability and/or safety of drugs; reported epidemiological findings; reported risk factors of APL or therapy of APL; reported a meta-analysis; or was a review article.
Data extraction and statistical analysis
Data regarding the demographic and clinical characteristics of the study subjects, trial endpoints, outcomes, and baseline values of related hematological and serological markers along with other clinical characteristics were extracted from each study report and tabulated in datasheets. Percent CR, relapse, 5-year OS, and 5-year disease-free survival (DFS) rates were extracted and their respective standard errors were calculated. Random effects meta-analyses were performed to achieve inverse variance-weighted effect sizes. Subgroup analyses were carried out for various combinations of drugs. Endpoints of the present meta-analysis were CR rate, relapse rate, 5-year OS, and 5-year DFS.
For metaregression analyses, against each of dependent variables (percent CR and percent relapse rates), we tested several explanatory (independent) variables including number of patients in a study, year of publication, follow-up duration, age, gender, baseline white blood cells (WBC) count, baseline platelets count, time to achieve CR, and the number of combining drugs for induction or consolidation. Metaregression analyses were carried out under random effects model using restricted maximum likelihood method. A p ˂ 0.1 was considered to show a significant relationship. Statistical indices for heterogeneity assessment were tau2 and I2. Publication bias was assessed by examining the funnel plot asymmetry and estimating the number of missing studies with trim and fill method. All analyses were carried out with the Stata software (Version 12; Stata Corporation, College station, TX, U.S.A.).
Results
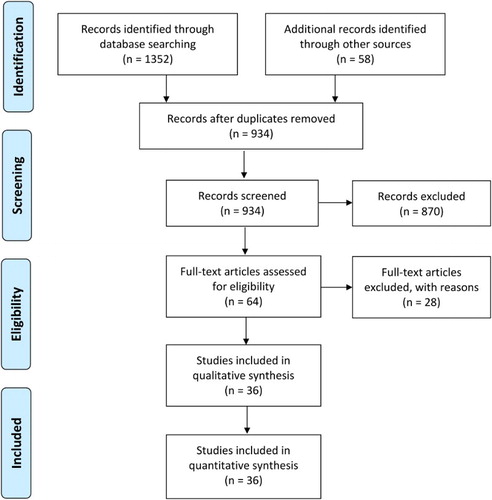
Thirty-seven studies [Citation23–59] including 15 RCTs, 7 prospective studies, and 15 retrospective studies fulfilled the eligibility criteria (). A significant publication bias was identified by the funnel plot symmetry tests (). Important characteristics of the included studies are presented in . Overall population of this meta-analysis consisted of 7566 newly diagnosed APL patients. Average age of the patients was 38.38 [95% confidence interval (CI): 33.31, 43.46] years and 48.65 [46.42, 50.88] % of the patients were male. Weighted WBC count was 1.55 [1.38, 1.72] (×109/l) and platelet count was 27.93 [25.17, 30.69] (×109/l).
Figure 2. A funnel plot showing outcomes of funnel plot symmetry test. Circles inside squares represent possible missing studies as identified by trim and fill method.
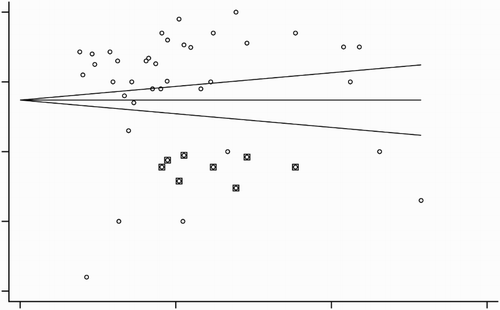
Table 1. Important characteristics of the included studies.
Median follow-up was 49.24 [95% CI: 41.33, 57.16] (range 12–121) months. For studies with longer follow-up, 5-year OS and DFS were 86.41 [95% CI: 83.97, 88.85] % and 75.42 [67.44, 83.40] %, respectively.
Pooled analysis of individual studies revealed that following induction therapy, 89.77 [95% CI: 87.04, 92.50] % patients achieved CR in the overall meta-analysis (). There were no significant differences in the overall CR rates with regard to study design (RCT, 92.1 [88.59, 95.61] %; prospective non-RCT, 90.21 [88.46, 91.96] %; and retrospective, 86.81 [80.07, 93.54] %). Complete remission was achieved in 38.25 [37.84, 38.65] days and 6.34 [5.98, 6.70] % of the patients died during induction.
Figure 3. Forest graph showing the overall effect size and subgroup-wise effect sizes of complete remission rate following various combined therapies for induction. Abbreviations: ATRA, all-trans retinoic acid; AraC, cytarabine; ATO, arsenic trioxide; BHAC, behenoyl cytosine arabinoside; DNR, daunorubicin; and IDA, idarubicin.
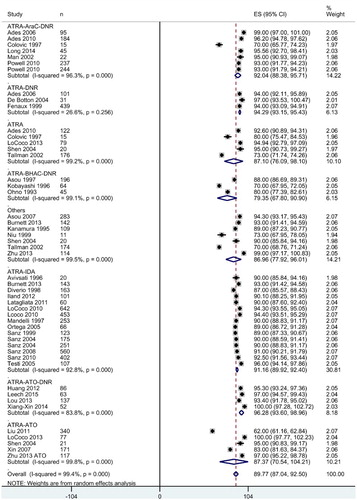
In subgroup analyses, the combined induction therapy with ATRA-ATO-DNR was associated with the highest rate of CR (96.28 [93.60, 98.96] %) followed by ATRA-DNR: 94.29 [93.15, 95.43] %, ATRA-AraC-DNR: 92.04 [88.38, 95.71] %, and ATRA-IDA: 91.16 [89.92, 92.40] % ().
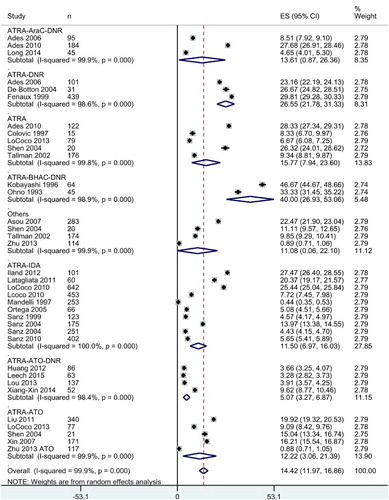
Overall relapse rate in the study population was 14.42 [11.97, 16.86] %. Induction therapy with ATRA-ATO-DNR was associated with least relapse rate (5.07 [3.27, 6.87] % followed by the ATRA-IDA combination (11.50 [6.97, 16.03] %; ).
Figure 4. Forest graph showing the overall effect size and subgroup-wise effect sizes of relapse rate following various combined therapies for induction. Abbreviations: ATRA, all-trans retinoic acid; AraC, cytarabine; ATO, arsenic trioxide; BHAC, behenoyl cytosine arabinoside; DNR, daunorubicin; and IDA, idarubicin.
For consolidation therapy, the number of drugs used in different studies was 1–6. However, there was no relationship between the number of drugs used for consolidation and OS. Although there was a positive relationship between the number of consolidation drugs and 5-year DFS, it could not achieve statistical significance in metaregression analysis (p = 0.160). Number of drugs used during maintenance period was also not associated with 5-year DFS.
In the metaregression analyses, WBC count was significantly negatively associated with CR rate (−0.608 [−1.25, 0.03]; p = 0.063). No other independent variable tested was significantly associated with CR. None of the independent variables tested was significantly associated with percent relapse rate (). However, there was a significant positive relationship between the year of publication (from 1990 to 2015) and CR rate (p < 0.00001; ) and a significant inverse relationship between the year of publication and relapse rate (p = 0.04).
Figure 5. A scatterplot showing the relationship between year of publication (from 1990 to 2015) and CR rate.
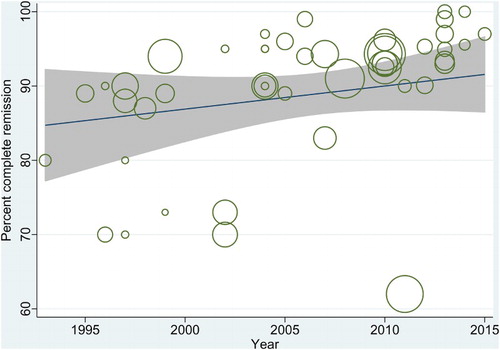
Table 2. Prognostic factor analysis for CR and relapse following combinational therapies to APL patients.
Discussion
In the present meta-analysis, we have observed that overall CR after combined chemotherapy for APL was higher with the use of ATRA-ATO-DNR followed by ATRA-DNR, ATRA-AraC-DNR, and ATRA-IDA combinations. Overall, CR was achieved in 41 ± 14 days and the induction mortality was approximately 6%. Overall relapse rate was 14%, but induction therapy with ATRA-ATO-DNR combination led to least relapse rate (5%) followed by the ATRA-IDA (11.5%). Five-year OS and DFS were 86% and 75%, respectively.
Among leukemias, APL is much sensitive to chemotherapy especially to ATRA which acts as a differentiation factor for the immature leukemic promyelocytes to be transformed into mature granulocytes [Citation60]. Higher efficacy of high-dose anthracycline in APL has been reported by earlier retrospective studies [Citation16,Citation61,Citation62]. Two most usual anthracyclines, IDA and DNR, are dose equivalent as 1:5 based on rodent studies [Citation63]. However, anthracycline use has been associated with significant cardiac toxicity [Citation64] and an increased risk of secondary malignancies [Citation65].
Addition of ATRA to chemotherapy results in rapid improvement in coagulopathy, bleeding severity, and blood product consumption [Citation66]. The use of ATRA has dramatically improved the survival outcomes of APL patients. It is a proapoptotic agent which sensitizes leukemia to chemotherapy and has improved the chances of cure to over 90% if treatment remains incident-free in the first 2 weeks. More recent achievement in APL chemotherapy is the use of ATO with ATRA which is found effective for induction as well as consolidation especially for patients who fail to achieve or sustain molecular response [Citation28]. The use of ATRA-ATO combination has been found to be less toxic regimen for APL. Standard induction therapy with anthracycline and ATRA, followed by a single cycle of ATO-based time-sequential consolidation therapy, is worth considerable for high-risk APL patients and for patients not willing to comply with longer treatments [Citation38]. Moreover, the use of ATO and AraC as prophylactic agents has also resulted in better outcomes. Central nervous system is the major site of extramedullary relapse in APL [Citation67].
Indeed, ATO exerts dual effects on APL cells in a dose-dependent manner. At low concentrations, ATO promotes APL cell differentiation, whereas at relatively higher concentrations, it induces apoptosis [Citation68]. In this meta-analysis, we have observed highest CR and lowest relapse rate with ATRA-ATO-anthracycline combination for induction. Thus, avenues should be explored to reduce anthracycline use in order to avoid their toxic effects.
Initial leukocyte count of ≥10 × 109 [Citation4,Citation69], higher circulating blast count, ATRA syndrome, and older age are reported to predict poor prognosis independently in APL patients [Citation70]. However, Lou et al. [Citation71] reported that high WBC count did not affect the remission-free survival of APL patients receiving ATO-based therapy. In the present study, we have found that baseline WBC count had a significant inverse relationship with CR rate but not with relapse rate. Moreover, age was also not found to be associated with prognosis in the present study.
For the management of APL, along with optimal treatment, timely diagnosis and supportive care also play a significant role in improving the outcomes. In the year 2005, a network of institutions, The International Consortium on APL, was established in developing countries in collaboration with well-reputed US and European cooperative groups to formulate expeditious diagnostic, treatment, and supportive guidelines. This networking improved the outcomes to bring the prognosis comparable to that of developed countries. Complete hematological remission rate reached to 85% and 2-year cumulative incidence of relapse was reduced to 4.5% with improvements in OS and DFS of up to 80 and 91%, respectively [Citation72].
A proportion of APL patients have genetic variants with different fusion transcripts such as PLZF/ RAR-α7 or STAT5b/RAR-α8 coding for fusion proteins that makes individuals ATRA resistant to initial treatment. Others, such as FLT-3 mutations, particularly the internal tandem duplication mutations affect up to 40% of APL cases. These variants are reported to be associated with high WBC count, the bcr3 PML breakpoint, a higher death rate during induction, higher relapse, and lower survival rates which necessitate the identification of candidates for ATRA-based induction therapy [Citation73].
Among the limitations of the present study, higher statistical heterogeneity (between-study inconsistency) is an important consideration. There could be several sources of which clinical and methodological heterogeneity may explain it to some extent. A strong trend has been seen in the improvement of treatment outcomes in recent years. Thus, the outcomes of earlier and later studies may also have caused some impact on statistical heterogeneity. Publication bias observed in the present study could also affect overall outcomes. Moreover, it was not possible to assess the relative impacts of induction, maintenance, and consolidation therapies owing to the lack of adequate outcome assessment data.
Conclusion
With an overall CR rate of 90% achieved within 41 ± 14 days with combined chemotherapy for APL, 5-year OS and DFS are estimated at approximately 86% and 75, respectively. Induction with ATRA-ATO-DNR combination led to the highest CR rate (96%) followed by ATRA-anthracyclines (ATRA-DNR: 94% and ATRA-AraC-DNR: 92%). Moreover, with an overall relapse rate of 14%, induction therapy with ATRA-ATO-DNR has also been found to be associated with least relapse rate (5%) followed by the ATRA-IDA (11.5%).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798
- Ferrara F. Acute promyelocytic leukemia: what are the treatment options? Expert Opin Pharmacother. 2010;11(4):587–596. doi: 10.1517/14656560903505115
- Zhou GB, Zhao WL, Wang ZY, et al. Retinoic acid and arsenic for treating acute promyelocytic leukemia. PLoS Med. 2005;2:e12. doi: 10.1371/journal.pmed.0020012
- Asou N, Adachi K, Tamura U, et al. Analysis of prognostic factors in newly diagnosed patients with acute promyelocytic leukemia: the APL92 study of the Japan Adult Leukemia Study Group (JALSG). Cancer Chemother Pharmacol. 2001;48:S65–71. doi: 10.1007/s002800100308
- Tallman MS, Kwaan HC. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood. 1992;79:543–53.
- Avvisati G, Mele A, Stazi MA, et al. Epidemiology of acute promyelocytic leukemia in Italy. Ann Oncol. 1991;2:405–411. doi: 10.1093/oxfordjournals.annonc.a057974
- Rees D, Grimwade D, Langabeer S, et al. Influence of genetic predisposition to thrombosis on natural history of acute promyelocytic leukaemia. MRC Adult Leukaemia Working Party. Br J Haematol. 1997;96(3):490–2. doi: 10.1046/j.1365-2141.1997.d01-2063.x
- Falanga A, Rickles FR. Pathogenesis and management of the bleeding diathesis in acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16(3):463–82. doi: 10.1016/S1521-6926(03)00059-8
- Douer D, Preston-Martin S, Chang E, et al. High frequency of acute promyelocytic leukemia among Latinos with acute myeloid leukemia. Blood. 1996;87(1):308–13.
- Thuler LC, Pombo-de-Oliveira MS. Acute promyelocytic leukaemia is highly frequent among acute myeloid leukaemias in Brazil: a hospital-based cancer registry study from 2001 to 2012. Ann Hematol. 2017;96(3):355–62. doi: 10.1007/s00277-016-2846-0
- Ades L, Chevret S, De Botton S, et al. Outcome of acute promyelocytic leukemia treated with all trans retinoic acid and chemotherapy in elderly patients: the European group experience. Leukemia. 2005;19(2):230–3. doi: 10.1038/sj.leu.2403597
- Zhang L, Samad A, Pombo-de-Oliveira MS, et al. Global characteristics of childhood acute promyelocytic leukemia. Blood Rev. 2015;29(2):101–25. doi: 10.1016/j.blre.2014.09.013
- Drapkin RL, Gee TS, Dowling MD, et al. Prophylactic heparin therapy in acute promyelocytic leukemia. Cancer. 1978;41(6):2484–90. doi: 10.1002/1097-0142(197806)41:6<2484::AID-CNCR2820410659>3.0.CO;2-#
- Bernard J, Weil M, Boiron M, et al. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973;41(4):489–96.
- Rodeghiero F, Avvisati G, Castaman G, et al. Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia. A GIMEMA retrospective study in 268 consecutive patients. Blood. 1990;75(11):2112–7.
- Head D, Kopecky KJ, Weick J, et al. Effect of aggressive daunomycin therapy on survival in acute promyelocytic leukemia. Blood. 1995;86(5):1717–28.
- Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567–72.
- Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–8. doi: 10.1056/NEJM199710093371501
- Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J. 2015;5:e304. doi: 10.1038/bcj.2015.25
- Ades L, Sanz MA, Chevret S, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): acomparison of French-Belgian-Swiss and PETHEMA results. Blood. 2008;111:1078–84. doi: 10.1182/blood-2007-07-099978
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. http://handbook.cochrane.org/.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097
- Ades L, Chevret S, Raffoux E, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24(36):5703–10. doi: 10.1200/JCO.2006.08.1596
- Adès L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115(9):1690–6. doi: 10.1182/blood-2009-07-233387
- Asou N, Adachi K, Tamura J, et al. All-trans retinoic acid therapy for newly diagnosed acute promyelocytic leukemia: comparison with intensive chemotherapy. The Japan Adult Leukemia Study Group (JALSG). Cancer Chemother Pharmacol. 1997;40(Suppl.):S30–e15. doi: 10.1007/s002800051058
- Asou N, Kishimoto Y, Kiyoi H, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110(1):59–66. doi: 10.1182/blood-2006-08-043992
- Avvisati G, Lo Coco F, Diverio D, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: A Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) pilot study. Blood. 1996;88(4):1390–1398.
- Burnett AK, Hills RK, Grimwade D, et al. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: results of the MRC AML15 trial. Leukemia. 2013;27(4):843–851. doi: 10.1038/leu.2012.360
- Colovic MD, Jankovic GM, Elezovic I, et al. Effect of all-trans-retinoic add alone or in combination with chemotherapy in newly diagnosed acute promyelocytic leukaemia. Med Oncol. 1997;14:65–72. doi: 10.1007/BF02990950
- de Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22(8):1404–1412. doi: 10.1200/JCO.2004.09.008
- Diverio D, Rossi V, Avvisati G, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of the PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter “AIDA” trial. GIMEMA-AIEOP Multicenter “AIDA” Trial. Blood. 1998;92(3):784–789.
- Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94(4):1192–1200.
- Huang H, Qin Y, Xu R, et al. Combination therapy with arsenic trioxide, all-trans retinoic acid, and chemotherapy in acute promyelocytic leukemia patients with various relapse risks. Leuk Res. 2012;36(7):841–845. doi: 10.1016/j.leukres.2012.03.027
- Iland H, Bradstock K, Seymour J, et al. Results of the APML3 trial incorporating all-trans-retinoic acid and idarubicin in both induction and consolidation as initial therapy for patients with acute promyelocytic leukemia. Haematologica. 2012;97(2):227–234. doi: 10.3324/haematol.2011.047506
- Kanamaru A, Takemoto Y, Tanimoto M, et al. All-trans retinoic acid for the treatment of newly diagnosed acute promyelocytic leukemia. Japan Adult Leukemia Study Group. Blood. 1995;85(5):1202–1206.
- Kobayashi T, Miyawaki S, Tanimoto M, et al. Randomized trials between behenoyl cytarabine and cytarabine in combination induction and consolidation therapy, and with or without ubenimex after maintenance/ intensification therapy in adult acute myeloid leukemia. J Clin Oncol. 1996;14:204–209. doi: 10.1200/JCO.1996.14.1.204
- Latagliata R, Breccia M, Fazi P, et al. GIMEMA AIDA 0493 amended protocol for elderly patients with acute promyelocytic leukaemia. Long-term results and prognostic factors. Br J Haematol. 2011;154(5):564–568. doi: 10.1111/j.1365-2141.2011.08593.x
- Leech M, Morris L, Stewart M, et al. Real-life experience of a brief arsenic trioxide-based consolidation chemotherapy in the management of acute promyelocytic leukemia: favorable outcomes with limited anthracycline exposure and shorter consolidation therapy. Clin Lymphoma Myeloma Leuk. 2015;15(5):292–297. doi: 10.1016/j.clml.2014.11.001
- Liu YJ, Wu DP, Liang JY, et al. Long-term survey of outcome in acute promyelocytic leukemia: a single center experience in 340 patients. Med Oncol. 2011;28(1):S513–S521. doi: 10.1007/s12032-010-9733-7
- Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–3179. doi: 10.1182/blood-2010-03-276196
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874
- Long ZJ, Hu Y, Li XD, et al. ATO/ATRA/anthracycline-chemotherapy sequential consolidation achieves long-term efficacy in primary acute promyelocytic leukemia. PLoS One. 2014;9(8):e104610). doi: 10.1371/journal.pone.0104610
- Lou Y, Qian q, Meng H, et al. High efficacy of arsenic trioxide plus all-trans retinoic acid based induction and maintenance therapy in newly diagnosed acute promyelocytic leukemia. Leukemia Res. 2013;37(1):37–42. doi: 10.1016/j.leukres.2012.09.004
- Mandelli F, Diverio D, Avvisati G, et al. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Blood. 1997;90(3):1014–1021.
- Mann G, Reinhardt D, Ritter J, et al. Treatment with all-trans retinoic acid in acute promyelocytic leukemia reduces early deaths in children. Ann Hematol. 2001;80(7):417–422. doi: 10.1007/s002770100304
- Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–3324.
- Ohno R, Kobayashi T, Tanimoto M, et al. Randomized study of individualized induction therapy with or without vincristine, and of maintenance intensification therapy between 4 or 12 courses in adult acute myeloid leukemia. Cancer. 1993;71:3888–3894. doi: 10.1002/1097-0142(19930615)71:12<3888::AID-CNCR2820711216>3.0.CO;2-G
- Ortega JJ, Madero L, Martín G, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23(30):7632–7640. doi: 10.1200/JCO.2005.01.3359
- Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–3757. doi: 10.1182/blood-2010-02-269621
- Sanz MA, Martín G, González M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–1243. doi: 10.1182/blood-2003-07-2462
- Sanz MA, Martín G, Rayón C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RAR alpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94(9):3015–3021.
- Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–5146. doi: 10.1182/blood-2010-01-266007
- Sanz MA, Montesinos P, Vellenga E, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112(8):3130–3134. doi: 10.1182/blood-2008-05-159632
- Shen ZX, Shi ZZ, Fang J, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2004;101(15):5328–5335. doi: 10.1073/pnas.0400053101
- Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–4302. doi: 10.1182/blood-2002-02-0632
- Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOPAIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106(2):447–453. doi: 10.1182/blood-2004-05-1971
- Xiang-Xin L, Lu-Qun W, Hao L, et al. Clinical study on prospective efficacy of all-trans acid, realgar-indigo naturalis formula combined with chemotherapy as maintenance treatment of acute promyelocytic leukemia. Evid Based Complement Alternat Med. 2014;2014:987560. doi: 10.1155/2014/987560
- Xin L, Wan-jun S, Zeng-jun L, et al. A survival study and prognostic factors analysis on acute promyelocytic leukemia at a single center. Leuk Res. 2007;31(6):765–771. doi: 10.1016/j.leukres.2006.07.028
- Zhu H, Wu D, Jin J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. 2013;31(33):4215–4222. doi: 10.1200/JCO.2013.48.8312
- Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20:57–65. doi: 10.1016/j.beha.2006.11.002
- Fenaux P, Pollet J, Vandenbossche L, et al. Treatment of acute promyelocytic leukemia: a report on 70 cases. Leuk Lymphoma. 1991a;4:249–253. doi: 10.3109/10428199109068072
- Fenaux P, Tertian G, Castaigne S, et al. A randomized trial of amsacrine and rubidazone in 39 patients with acute promyelocytic leukemia. J Clin Oncol. 1991b;9:1556–1561. doi: 10.1200/JCO.1991.9.9.1556
- Casazza AM, Pratesi G, Giuliani F, et al. Antileukemic activity of 4-demethoxydaunorubicin in mice. Tumor. 1980;66:549–564.
- Thomas X, Le Q, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol. 2002;81:504–507. doi: 10.1007/s00277-002-0534-8
- Garcia-Manero G, Kantarjian HM, Kornblau S, et al. Therapy-related myelodysplastic syndrome or acute myelogenous leukemia in patients with acute promyelocytic leukemia (APL). Leukemia. 2002;16:1888–1893. doi: 10.1038/sj.leu.2402616
- Di Bona E, Avvisati G, Castaman G, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leukaemia. Br J Haematol. 2000;108(4):689–695. doi: 10.1046/j.1365-2141.2000.01936.x
- Park J, Jurcic JG, Rosenblat T, et al. Emerging new approaches for the treatment of acute promyelocytic leukemia. Ther Adv Hematol. 2011;2(5):335–352. doi: 10.1177/2040620711410773
- Zhou GB, Zhang J, Wang ZY, et al. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy. Philos Trans R Soc Lond B Biol Sci. 2007;362:959–971. doi: 10.1098/rstb.2007.2026
- Sanz MA, Lo Coco F, Martín G, et al. Definition of relapse risk and role of non-anthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–1253.
- De Botton S, Dombret H, Sanz M, et al. Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1998;92(8):2712–2718.
- Lou Y, Ma Y, Suo S, et al. Prognostic factors of patients with newly diagnosed acute promyelocytic leukemia treated with arsenic trioxide-based frontline therapy. Leuk Res. 2015;39(9):938–944. doi: 10.1016/j.leukres.2015.05.016
- Rego EM, Kim HT, Ruiz-Argüelles GJ, et al. Improving acute promyelocytic leukemia (APL) outcome in developing countries through networking, results of the International Consortium on APL. Blood. 2013;121(11):1935–1943. doi: 10.1182/blood-2012-08-449918
- Baljevic M, Park JH, Stein E, et al. Curing all patients with acute promyelocytic leukemia: are we there yet? Hematol Oncol Clin North Am. 2011;25(6):1215–1233. doi: 10.1016/j.hoc.2011.10.002