ABSTRACT
Background and aims: Vitamin D deficiency and increased platelet indices are associated with increased rate or risk of several diseases such as cardiovascular disease and metabolic syndrome, respectively. We investigated whether vitamin D deficiency is associated with increased platelet count (PC) and mean platelet volume (MPV).
Methods and results: The study included 3190 subjects older than 20 years. Subjects were divided into three groups based on their vitamin D levels: vitamin D deficiency (<10.0 ng/ml); insufficiency (10–20 ng/ml); and sufficiency (>20.0 ng/ml). The associations between platelet indices and various parameters were analyzed by Pearson’s correlation analysis and t-tests. Then, multivariate linear regression analyses were done correcting for associated parameters. PC and MPV showed a negative correlation with vitamin D groups by ANOVA and multiple linear regression. PC was inversely related with vitamin D group after adjusting for sex, age, regular exercise, white blood cell count, total cholesterol, hemoglobin, and creatinine levels (β ± SE = −3.461 ± 1.512, P = 0.022). MPV was also inversely related with vitamin D group after adjusting for regular exercise, hemoglobin level, and total cholesterol level (β ± SE = −0.080 ± 0.026, P = 0.002), and this relationship remained statistically significant after adjusting for regular exercise, hemoglobin level, total cholesterol level, diabetes, hypertension, and body mass index (β ± SE=−0.082 ± 0.026, P = 0.002).
Conclusion: PC and MPV are inversely associated with vitamin D levels in adults.
Introduction
Vitamin D is a type of secosteroid hormone with two representative forms: vitamin D2 (ergocalciferol; 25(OH)D2) and vitamin D3 (cholecalciferol; 25(OH)D3). Vitamin D2 comes from ergosterol in foods or supplements, while vitamin D3 is produced from previtamin D3 that is derived from 7-dehydroxycholesterol upon exposure of the skin to ultraviolet light. Vitamin D has significant roles in calcium homeostasis, bone mineralization [Citation1], immunity [Citation2], cell proliferation and differentiation [Citation3], anticoagulation [Citation4], and anti-inflammation [Citation5].
Platelets are small but active anucleate cell fragments that originate from megakaryocytes in the bone marrow, and function in hemostasis and thrombosis [Citation6], immunity [Citation7], inflammation [Citation8], and atherosclerosis [Citation9]. Platelet count (PC) and platelet size are representative laboratory measures of platelets, and are widely used as indicators of the normal inflammatory reaction against infection [Citation10].
Mean platelet volume (MPV) is a measure of the average size of platelets and serves as an index of platelet activation, which connects thrombosis and inflammation [Citation11]. Larger macrothrombocytes, as reflected by a larger MPV, are hemostatically more reactive and thrombogenic than smaller ones [Citation12].
Over the past few decades, a considerable numbers of studies have investigated the associations between vitamin D level, PC, and MPV with several diseases. Both vitamin D deficiency and elevated MPV are associated with increased rate or risk of coronary artery disease [Citation13,Citation14], stroke [Citation15,Citation16], metabolic syndrome [Citation17,Citation18], obesity [Citation19,Citation20], hypertension [Citation21,Citation22], and type 2 diabetes [Citation23,Citation24], but few attempts have been made to determine the relationship between vitamin D and MPV [Citation4,Citation25]. Here, we investigated the relationship between changes in MPV and vitamin D levels in Korean adults.
Methods
Study population
We recruited 3625 Koreans who visited the Health Promotion Center of Wonju Severance Christian Hospital for a regular health examination from 2 March 2012 to 31 December 2012. Subjects who had a past medical history of coronary artery disease, stroke, or chronic liver disease were excluded. Subjects who had the possibility of an inflammatory disorder, infection, anemia, bone marrow suppressive disorder indicated by a WBC count in excess of 10.0 × 103 cells/μl or less than 3.0 × 103 cells/μl, PC less than 150 × 103/μl or more than 400 × 103/μl, hemoglobin less than 12.0 g/dl in females and less than 13.0 g/dl in males, and C-reactive protein (CRP) level in excess of 10.0 mg/l were also excluded. A total of 3190 subjects were included in the final statistical analysis.
Study design
Medical history and anthropometrics
All subjects provided informed consent. This study was reviewed and approved by the Institutional Review Board of Wonju Severance Christian Hospital, Yonsei University College of Medicine in Wonju, Korea. We administered a self-report questionnaire to obtain medical history, medication, smoking status, alcohol ingestion, and exercise habit data from all participants. Subjects were divided into two groups according to smoking status: current smokers and non-smokers. Non-smoker group included subjects who had quit smoking 6 months prior. Alcohol ingestion was determined as drinking regularly more than once a week. Regular exercise was defined as engaging in moderate intensity exercise for more than 30 minutes at least three times a week. We measured systolic blood pressure (SBP) and diastolic blood pressure (DBP). Body mass index (BMI), the ratio of body weight in kilograms to the square of its height in meters, was calculated as a measure of body fat.
Definition of vitamin D sufficiency, insufficiency, deficiency
Biochemical measurements
Blood pressure was measured after subjects had rested for more than 5 minutes. All blood samples were collected from the antecubital vein after fasting for 12 hours. White blood cell count, hemoglobin, PC, and MPV were analyzed using an automated blood cell counter (ADVIA 2120I, Bayer, NY, USA) within an hour of collection. Total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, fasting glucose, and CRP levels were analyzed with Modular DPE (Roche Diagnostics, Switzerland) and the vitamin D concentration by an immunoassay system (ADVIA Centaur XP, Siemens, NY, USA).
Statistical analysis
We used SPSS Windows version 21.0(18.0) (SPSS Inc., Chicago, IL, USA). All continuous variables were checked with Kolmogorov–Smirnov normality test to show their distributions. The continuous variables in all groups showed a normal distribution. Subjects were categorized into one of three groups according to their vitamin D level: deficiency (less than 10.0 ng/ml); insufficiency (10–20 ng/ml); and sufficiency (more than 20.0 ng/ml) [Citation26]. ANOVA was performed to determine the relationship between platelet indices and groups. Pearson’s correlation analysis and t-tests were performed to evaluate the associations between platelet indices and other variables. Then, the relationship between vitamin D group and PC, and between vitamin D group and MPV were evaluated through multiple linear regression analysis. All data are presented as the mean and standard deviation (SD) for continuous variables and as percentages for categorical variables. Statistical significance was defined as P < 0.05.
Results
General characteristics and simple analysis
Demographic, anthropometric, and biochemical characteristics of the study subjects according to vitamin D status are shown in . Mean age of participants was 45.0 ± 9.2, 45.0 ± 8, and 47.6 ± 9.5 in vitamin D deficiency, insufficiency, and sufficiency groups, respectively. More females than males were vitamin D deficient. Fasting glucose and triglyceride levels had inverse correlations with vitamin D levels.
Table 1. Characteristics of subjects according to deficiency, insufficiency, and sufficiency of vitamin D.a
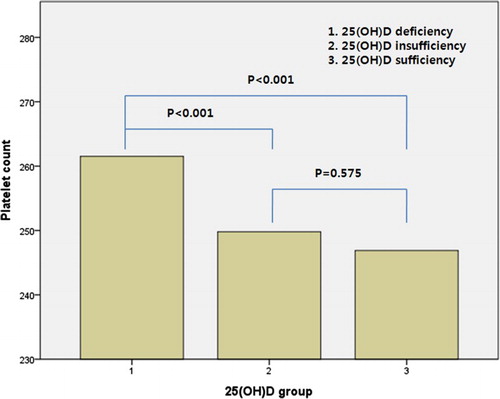
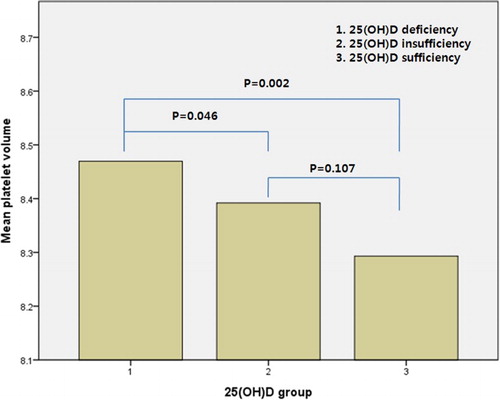
ANOVA and the χ2 test showed a negative correlation between vitamin D group and platelet indices. Mean PC was highest in those patients in the deficiency group (deficiency; 261.5 ± 51.2, insufficiency; 249.8 ± 47.6 and sufficiency; 246.9 ± 47.4, P < 0.001) and MPV showed the same tendency (deficiency; 8.5 ± 0.8, insufficiency; 8.4 ± 0.8 and sufficiency; 8.3 ± 0.8, P < 0.001) ().
The vitamin D insufficiency and sufficiency groups had a significantly lower PC and MPV than the deficiency group ( and ).
Relationship between platelet indices and subject characteristics
PC had a positive correlation with WBCs, total cholesterol level, triglyceride level, and CRP level, and a negative correlation with age, hemoglobin level, and creatinine level (P < 0.05). MPV was negatively correlated with hemoglobin and total cholesterol levels (P < 0.05). There were no statistically significant associations between BMI and PC and MPV (). PC showed negative association with MPV ().
Figure 3. The expected inverse relationship between platelet count and mean platelet volume. P-value was calculated by Pearson’s correlation.
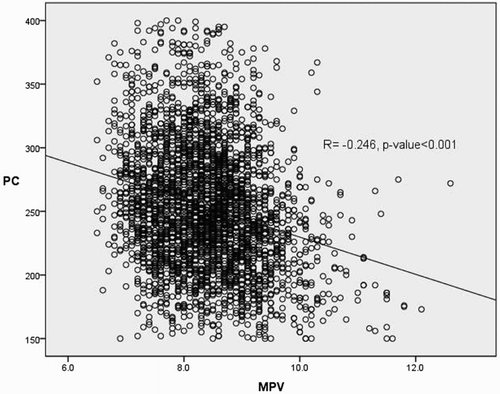
Table 2. Correlations between platelet indices and continuous variables (Pearson’s correlation).
Sex and regular exercise were associated with PC based on t-tests. However, only regular exercise was significantly associated with MPV ().
Table 3. Correlations between platelet indices and nominal variables (t-tests).
Relationship between vitamin D level and platelet count
PC was independently and inversely related with vitamin D groups in multiple linear regression analyses, and same correlation tendency remained after adjusting for age and sex in Model 2. Multivariate analyses after adjustment for sex, age, regular exercise, WBC count, total cholesterol level, hemoglobin level, creatinine level, and CRP level were conducted in Model 3, as these factors were correlated with PC in previous analyses. MPV and vitamin D groups were shown to be inversely associated ().
Table 4. Multiple linear regression analyses showing the independent contribution of vitamin D group to platelet count.
Relationship between vitamin D levels and MPV
MPV was also independently and inversely related with vitamin D groups based on multiple linear regression analyses. This association remained after adjusting for regular exercise, hemoglobin level, and total cholesterol level in Model 2. Multivariate analyses after adding DM, HTN, and BMI variables, which are known to be related to MPV, showed a statistically significant negative correlation ().
Table 5. Multiple linear regression analyses showing the independent contribution of vitamin D group to mean platelet volume.
Discussion
MPV was inversely related with vitamin D level in our cross-sectional study. This association remained significant even after adjusting for regular exercise, hemoglobin level, total cholesterol level, DM, HTN, and BMI.
Kebapcilar et al. [Citation25] suggested that vitamin D deficiency plays an important role in primary ovarian insufficiency (POI) in women and is associated with coagulation, independent of age and BMI. Furthermore, POI patients had elevated MPV and their serum vitamin D levels were inversely correlated with MPV, consistent with our findings.
There are several possible explanatory mechanisms for the inverse association of MPV with vitamin D deficiency. Vitamin D has anti-thrombogenic, anti-inflammatory, and anticoagulation activity, while MPV links thrombosis and inflammation.
Aihara et al. [Citation27] demonstrated that the vitamin D-vitamin D receptor (VDR) system may play an important role in anti-thrombogenicity in vivo by showing that VDR knock-out (VDRKO) mice manifested exacerbated multi-organ thrombus formation after exogenous lipopolysaccharide injection, regardless of calcemic conditions. Activated vitamin D up-regulates the gene expression of antithrombotic factors (ATs) and thrombomodulin in monocytic cells, whereas it down-regulates thrombogenic factor (TF) gene expression. In VDRKO mice, the reverse was observed. Thus, the vitamin D-VDR system enhances the expression of ATs while inhibiting the expression of TFs.
Vitamin D suppresses activation of TNF-alpha-converting enzyme (TACE) in renal cells by inhibiting release of mediators of systemic inflammation, namely tumor necrosis factor alpha (TNF-alpha), intercellular adhesion molecule 1 (ICAM-1), and vascular adhesion molecule 1 (VCAM-1), into the circulation [Citation28], which explains its anti-inflammatory effects. Also, TNF-alpha and IL-6 decreased by vitamin D therapy [Citation29], are related to oxidative stress and stimulate megakaryopoiesis [Citation30].
Vitamin D has also been shown to have anticoagulant effects on monocytic leukemia cells and monocytes, analogous to retinoic acids [Citation4].
Gasparyan et al. [Citation11] showed that MPV reflects both proinflammatory and prothrombotic conditions, where thrombopoietin and numerous inflammatory cytokines such as IL-1, IL-6, and TNF-alpha regulate thrombopoiesis. MPV is also dependent on the intensity of systemic inflammation.
Consequently, vitamin D deficiency increases release of these cytokines to regulate thrombopoiesis and inflammation, and thrombosis and inflammation result in increased MPV. We confirmed in our study that MPV was inversely related with vitamin D levels in both genders.
We also found that PC was inversely related to vitamin D levels in this study. No previous studies have clearly demonstrated increased PC in patients with vitamin D deficiency. These observed associations may be due to the close relationship between oxidative stress and platelet count.
Vitamin D’s role as an antioxidant is well known [Citation31–33]. There are several studies that have demonstrated that oxidative stress causes thrombocytosis, such as that of Ishii et al. [Citation34] in mice. A positive association between oxidative stress and a chronic low-grade inflammation index including platelet count has also been shown in the general population [Citation35]. In addition, a population-based cohort study reported that anti-oxidation is associated with lower platelet count [Citation36]. Furthermore, previous studies have shown that elevated antioxidant levels are associated with inhibition of vascular endothelial growth factor release [Citation37] and lower inflammation [Citation38].
Our study had several limitations. First, it was a cross-sectional study, therefore we could not infer causality. Second, there may have been selection bias because the study population, who were healthy enough to visit a hospital for a regular health examination, may not be representative of all Korean adults. Last, we used blood sampling tubes containing EDTA, which can cause time-dependent swelling of platelets. To prevent platelet swelling, we analyzed all blood samples within an hour [Citation39].
However, to our knowledge, this is the first study to define the relationship between vitamin D levels and PC in the general population. Moreover, very few studies have explored the association between vitamin D level and MPV.
In conclusion, we showed that MPV was inversely associated with vitamin D level. This result indicates that maintenance of an elevated vitamin D level in vivo is important because it is associated with a lower MPV, which in turn is related to a lower risk of diseases such as atherosclerosis. However, further studies are needed to clarify the mechanisms by which vitamin D level affects MPV or vice versa and to test the hypothesis that vitamin D supplementation may decrease MPV.
Acknowledgements
We express our special thanks to Dr Juwon Kim who gave a lot of advice and attended a discussion.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anderson PH, Turner AG, Morris HA. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin Biochem. 2012;45(12):880–886. doi: 10.1016/j.clinbiochem.2012.02.020
- Bikle DD. Vitamin D regulation of immune function. Vitam Horm. 2011;86:1–21. doi: 10.1016/B978-0-12-386960-9.00001-0
- Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66(10):S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x
- Koyama T, Hirosawa S. Anticoagulant effects of synthetic retinoids and activated vitamin D3. Semin Thromb Hemost. 1998;24(3):217–226. doi: 10.1055/s-2007-995845
- Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611
- Wang Y, Andrews M, Yang Y, et al. Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc Hematol Disord Drug Targets. 2012;12(2):126–132. doi: 10.2174/1871529X11202020126
- Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67(4):499–511. doi: 10.1007/s00018-009-0205-1
- Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112(11):1506–1519. doi: 10.1161/CIRCRESAHA.113.300512
- Li NL. CD4(+) t cells in atherosclerosis: regulation by platelets. Thromb Haemost. 2013;109(6):980–990. doi: 10.1160/TH12-11-0819
- Prina E, Ferrer M, Ranzani OT, et al. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest. 2013;143(3):767–775. doi: 10.1378/chest.12-1235
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47–58. doi: 10.2174/138161211795049804
- Yuce M, Cakici M, Davutoglu V, et al. Relationship between mean platelet volume and atrial thrombus in patients with atrial fibrillation. Blood Coagul Fibrin. 2010;21(8):722–725.
- de Boer IH, Kestenbaum B, Shoben AB, et al. 25-Hydroxyvitamin d levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20(8):1805–1812. doi: 10.1681/ASN.2008111157
- Jung DH, Lee HR, Lee YJ, et al. The association between coronary artery calcification and mean platelet volume in the general population. Platelets. 2011;22(8):567–571. doi: 10.3109/09537104.2011.580397
- Ghahremanfard F, Asghari N, Ghorbani R, et al. The relationship between mean platelet volume and severity of acute ischemic brain stroke. Neurosciences (Riyadh, Saudi Arabia). 2013;18(2):147–151.
- Sun Q, Pan A, Hu FB, et al. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43(6):1470–1477. doi: 10.1161/STROKEAHA.111.636910
- Gagnon C, Lu ZX, Magliano DJ, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian diabetes, obesity and lifestyle study: AusDiab). J Clin Endocr Metab. 2012;97(6):1953–1961. doi: 10.1210/jc.2011-3187
- Tavil Y, Sen N, Yazici HU, et al. Mean platelet volume in patients with metabolic syndrome and its relationship with coronary artery disease. Thromb Res. 2007;120(2):245–250. doi: 10.1016/j.thromres.2006.10.005
- Coban E, Ozdogan M, Yazicioglu G, et al. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59(8):981–982. doi: 10.1111/j.1742-1241.2005.00500.x
- Gonzalez-Molero I, Rojo-Martinez G, Morcillo S, et al. Hypovitaminosis D and incidence of obesity: a prospective study. Eur J Clin Nutr. 2013;67(6):680–682. doi: 10.1038/ejcn.2013.48
- Ullah MI, Uwaifo GI, Nicholas WC, et al. Does vitamin D deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int J Endocrinol. 2010; 2010(579640):1–11. doi: 10.1155/2010/579640
- Varol E, Akcay S, Icli A, et al. Mean platelet volume in patients with prehypertension and hypertension. Clin Hemorheol Microcirc. 2010;45(1):67–72.
- Gonzalez-Molero I, Rojo-Martinez G, Morcillo S, et al. Vitamin D and incidence of diabetes: a prospective cohort study. Clin Nutr. 2012;31(4):571–573. doi: 10.1016/j.clnu.2011.12.001
- Hekimsoy Z, Payzin B, Ornek T, et al. Mean platelet volume in type 2 diabetic patients. J Diabetes Complicat. 2004;18(3):173–176. doi: 10.1016/S1056-8727(02)00282-9
- Kebapcilar AG, Kulaksizoglu M, Ipekci SH, et al. Relationship between mean platelet volume and low-grade systemic coagulation with vitamin D deficiency in primary ovarian insufficiency. Arch Gynecol Obstet. 2013;288(1):207–212. doi: 10.1007/s00404-013-2735-x
- McKenna MJ, Freaney R. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Osteoporosis Int. 1998;8:S3–S6. doi: 10.1007/PL00022725
- Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279(34):35798–35802. doi: 10.1074/jbc.M404865200
- Querfeld U. Vitamin D and inflammation. Pediatr Nephrol. 2013;28(4):605–610. doi: 10.1007/s00467-012-2377-4
- Luo J, Wen H, Guo H, et al. 1,25-dihydroxyvitamin D3 inhibits the RANKL pathway and impacts on the production of pathway-associated cytokines in early rheumatoid arthritis. Biomed Res Int. 2013;2013(101805):1–9.
- Cure E, Balik MS, Cumhur Cure M, et al. Is the mean platelet volume predictive of hip fractures in the elderly? Ann Lab Med. 2013;33(5):367–370. doi: 10.3343/alm.2013.33.5.367
- Peery SL, Nemere I. Contributions of pro-oxidant and anti-oxidant conditions to the actions of 24,25-dihydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on phosphate uptake in intestinal cells. J Cell Biochem. 2007;101(5):1176–1184. doi: 10.1002/jcb.21238
- Polidoro L, Properzi G, Marampon F, et al. Vitamin D protects human endothelial cells from H(2)O(2) oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6(2):221–231. doi: 10.1007/s12265-012-9436-x
- Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, et al. Role of vitamins D, E and C in immunity and inflammation. J Biol Regul Homeost Agents. 2013;27(2):291–295.
- Ishii T, Miyazawa M, Takanashi Y, et al. Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion. Redox Biol. 2014;2:679–685. doi: 10.1016/j.redox.2014.05.001
- Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446
- Bonaccio M, Di Castelnuovo A, De Curtis A, et al. Adherence to the Mediterranean diet is associated with lower platelet and leukocyte counts: results from the Moli-sani study. Blood. 2014;123(19):3037–3044. doi: 10.1182/blood-2013-12-541672
- Schindler R, Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J Nutr. 2006;136(6):1477–1482.
- Scoditti E, Calabriso N, Massaro M, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527(2):81–89. doi: 10.1016/j.abb.2012.05.003
- Dastjerdi MS, Emami T, Najafian A, et al. Mean platelet volume measurement, EDTA or citrate? Hematology (Amsterdam, the Netherlands). 2006;11(5):317–319.