ABSTRACT
Objectives: Impaired platelet production has been found to be an important pathological mechanism of thrombocytopenia in many diseases. Platelet generation is a complex process that mainly occurs in the bone marrow, and thus is closely regulated by the bone marrow microenvironment. This review attempts to summarize the most current knowledge referring the role of bone marrow microenvironment in the regulation of platelet production.
Methods: The effects of multiple microenvironment ingredients in regulating megakaryopoiesis and thrombocytopoiesis have been discussed. Abnormalities of these components in thrombocytopenic diseases are also described.
Discussions: Thrombocytopenia is a common clinical manifestation of a variety of diseases. The functional importance of platelets has driven the developments of a broad range of studies. Platelet generation mainly occurs within the bone marrow, where the cells, soluble factors, and extracellular matrix proteins collaboratively form a complex regulatory network, directing megakaryocytic proliferation and differentiation. Alteration in any part of the regulating network may result in defective platelet formation, and eventually lead to thrombocytopenia. A variety of thrombocytopenic diseases have been found to be related with the disregulated bone marrow microenvironment. Identification of the variations of these niche ingredients in certain diseases has facilitated the developments of multiple therapeutic regimes. Further studies that can combine these niche factors with their downstream regulatory factors will be beneficial for developing more effective therapies.
Conclusions: Further definition of the role of bone marrow microenvironment in platelet generation may deepen our understanding of the underlying mechanisms as well as provide new therapeutic targets for thrombocytopenic diseases.
Introduction
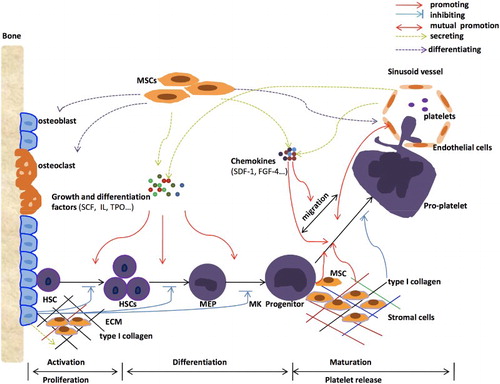
Platelets in the peripheral blood play essential roles in multiple physiological activities, including hemostasis, immune responses, inflammation, and thrombosis [Citation1]. Thrombocytopenia is a common clinical manifestation of a variety of diseases. The functional importance of platelets has driven the developments of a broad range of studies. In adults, the physiological process of platelet production mainly occurs within the bone marrow, involving a series of cellular events, beginning with hematopoietic stem cells (HSCs) proliferation and ending with the generation of functional platelets, so as to satisfy physiological requests [Citation2]. It is clear that as the site where platelet generation occurs, the bone marrow microenvironment (BMM) plays the critical role in orchestrating the whole process. However, the composition and function of each niche, and the definite regulation mechanism are still ill-defined. As has already known, the BMM is mainly composed of non-hematopoietic cells, soluble factors, and extracellular matrix proteins, which collaboratively form a complex regulatory network, directing hematopoietic cells proliferation and differentiation [Citation3]. Meanwhile, abnormal expressions of any of these components may have great influences on the processes of hematopoiesis and lead to fluctuations of the peripheral blood cells. Recently, several studies have illustrated that some of these components are indispensable in specific differentiation and proliferation stages of platelet generation, and a variety of thrombocytopenic diseases have been found to be related with the disregulated BMM, including idiopathic thrombocytopenic purpura (ITP), congenital thrombocytopenia, aplastic anemia (AA) and so on [Citation4–Citation6]. In this review, we first briefly introduce the concept of bone marrow niche and summarize the basic process of platelet generation in the bone marrow. Then, we review some of the ingredients of the BMM that have already been proved critical for megakaryopoiesis or thrombopoiesis. We classify these components into soluble factors (), supportive cells and extracellular matrix (ECM) to discuss their specific roles respectively, and illustrate their abnormalities in certain thrombocytopenic diseases. In addition, the bone marrow physical microenvironment for the regulation of platelet formation has also be discussed.
Table 1. Soluble factors in the bone marrow microenvironment that essential for platelet production.
Bone marrow hematopoietic niche and the basic process of platelet generation
Platelets are directly generated from megakaryocytes (MKs), which are in fact derived from HSCs, the common progenitors of all lineage blood cells [Citation7]. Within the bone marrow, HSCs reside in a specific region, where they are surrounded by various non-hematopoietic components, including multiple supporting cells, soluble factors, extracellular matrix proteins, nervous system, oxygen, and so on [Citation8Citation9–Citation10]. These non-hematopoietic components collaboratively form a hematopoietic microenvironment, which is essential in determining stem cell fate with regard to self-renewal versus differentiation [Citation11]. The concept ‘niche’ was first proposed to describe the physiological microenvironment of HSCs where they reside [Citation12]. Now it is widely recognized that niche is actually a regulatory system that can control HSCs’ activities from their surroundings, and thus is vital in regulating a series of cellular events [Citation13]. Discriminated by their localization, cellular compositions, and hematopoiesis regulatory effects, there are at least two kinds of niches that have already been identified for hematopoiesis, including the osteoblastic niche and the vascular niche [Citation11]. Accordingly, the osteoblastic niche lining of the endosteal surface is mainly composed of osteoblasts and osteolineage cells [Citation14], where HSCs are maintained in a quiescent state for self-renewal while inhibited from terminal differentiation [Citation15]. Whereas, the vascular niche, comprising of vascular endothelial cells, mesenchymal stem cells (MSCs), CXCL12-abundant reticular (CAR) cells [Citation11], is the site for the proliferation, differentiation and mobilization of HSCs [Citation16]. Under physiological conditions, the coordinated work between this two kinds of niches control the normal processes of cell differentiation and development in the bone marrow. Meanwhile, the process of platelet production, including MK differentiation, pro-platelet formation (PPF) and platelet release, is also closely correlated with this two kinds of niches.
The classical model of platelet production used to be concluded as a 2-stage process entailing the differentiation of HSCs into MKs, and then the maturation of MKs to generate functional platelets [Citation17]. As mentioned above, HSCs are maintained in a quiescent state by the environmental cues in the osteoblastic niche, which is regarded as an important mechanism to prevent the premature exhaustion of stem cells and so that to ensure lifelong hematopoiesis [Citation15] However, there are still a small percentage of HSCs that are continued to be activated by specific growth factors and differentiation factors, which will then get to proliferate and differentiate into multilineage blood cells [Citation18Citation19–Citation20]. To generate MKs, HSCs are first activated by factors like stem cell factors (SCF), interleukin (IL), and so on. Activated HSCs then begin to proliferate and undergo a series of lineage commitment steps, in which they progressively losing the ability for self-renewal while giving rise to increasingly committed progenitors, and after going through the multi-, bi-potent stages, they will eventually differentiate into unipotent megakaryocytic progenitors [Citation17]. Thrombopoietin (TPO) and many other soluble factors have been identified to play indispensable roles in mediating this specific differentiation process [Citation21]. After that, MKs will migrate from osteoblastic niche to vascular niche for further maturation and development, a process known to be mediated by specific chemokines and adhesion molecules. MK maturation is characterized by DNA replication, cytoplasmic maturation, and upregulation of the required components for generating functional platelets [Citation22]. The reorganization of MK cytoskeletal microtubule and actin, followed by the extension of cytoplasm to across the vascular endothelial, is a committed step to form pseudopodial elongations called pro-platelets [Citation23]. Pro-platelets are presented as platelet-sized swellings in tandem arrays that are connected by thin cytoplasmic bridges, which can further disintegrate into thousands of platelets [Citation24]. The mutual interaction between ECM and megakaryocytic cells is vital in coordinating these processes. This classical model reflects the basic process of platelet production, which is tightly regulated by the BMM. However, this classic model has also been questioned to be over simplistic, as the identification of some new lineage-biased progenitor populations [Citation2,Citation7,Citation20] as well as the discovery of some platelet generation patterns that need not pass through the pro-platelet stage [Citation17]. Results from all these findings suggest that platelet generation is extremely complicated, and the underlying mechanism is far from clear. However, what we can confirm is that platelet generation is closely correlated with the BMM. Meanwhile, problems in any of the aforementioned processes will certainly affect platelet production, and eventually lead to thrombocytopenia. Identification of the abnormal expressions of these specific BMM components that dedicate for platelet production in certain thrombocytopenic diseases will be of great importance for both the etiology researches as well as treatments of these diseases.
Critical soluble regulatory factors for megakaryopoiesis and thrombopoiesis
Growth and differentiation factors
Megakaryopoiesis and thrombopoiesis in the bone marrow are under the control of a complex network of hematopoietic cytokines, including SCF, granulocyte colony-stimulating factor (G-CSF), TPO, and interleukin family proteins (IL-3, IL-6, IL-11, etc.) [Citation25,Citation26]. Many lines of evidences have indicated that TPO, acting through its receptor c-Mpl, is the primary regulator of early lineage determination activities towards megakaryocytic series as well as the proliferation of committed megakaryocytic progenitors [Citation26Citation27 Citation28–Citation29]. Binding of TPO to c-Mpl can lead to receptor dimerization, activation of a wide range of intracellular signal transduction pathways, and eventually cell responses, means the proliferation and differentiation of megakaryocytic precursors [Citation27,Citation30]. The functional activity of TPO/c-Mpl is essential in maintaining MK and platelet counts. Previous studies have showed that mice with defects in either TPO or its receptor exhibited markedly reduced MKs and colony forming unit-megakaryocytes (CFU-MKs), and only retain 10–15% of the normal platelet levels [Citation28]. Kaushansky et al. [Citation31] also demonstrated that without TPO, CFU-MK is completely aborted in mice models. In humans, loss of function mutants of TPO or c-Mpl gene have been described to cause thrombocytopenia in diseases like congenital amegakaryocytic thrombocytopenia and AA [Citation21]. The autoantibody against TPO or its receptor has also been suggested to be a common pathogenic factor contributing to decreased platelet production in some of the ITP and systemic lupus erythematosus (SLE) patients [Citation21,Citation32]. Results from all these findings raise the possibility to utilize exogenous TPO analogues or receptor agonists to stimulate platelet production in certain thrombocytopenic patients, in those who are encountered with decreased intrinsic TPO level or impaired TPO/c-Mpl interaction. As we all known, TPO is mainly produced in the liver, which will be internalized and degraded upon binding with c-Mpl by those receptor-bearing cells. The serum level of TPO is determined by the balance between its production and destruction [Citation33]. In some thrombocytopenic patients, for example in AA, with megakaryocytic hypoplasia, little of the hepatic TPO production is absorbed by platelets, allowing blood level to rise. However, TPO levels are low when thrombocytopenia is due to increased platelet destruction with normal or increased MK mass, such as in ITP [Citation34]. Therefore, measuring TPO levels in patients may help to distinguish between hypoproliferative and consumptive thrombocytopenia. Furthermore, it can also serve as an indicator of when and where TPO analogues or receptor agonists are needed. For example, TPO analogues or receptor agonists often exert good effects in ITP patients, because the rate of platelet destruction can be overcome by physiologically attainable increases in production [Citation35]. In addition to constitutively expressed in the liver, the transcription of TPO gene can also be substantially induced in marrow stromal cells in response to thrombocytopenia. Inflammatory responses are also associated with increased TPO expression, which is found to be mediated by IL-6 [Citation36]. In conclusion, a great many of thrombocytopenic diseases are correlated with abnormal expression of TPO or c-Mpl gene, indicating that correct the dysregulated TPO/c-Mpl pathway, either by using exogenous TPO analogues or through inducing endogenous production, may become effective therapeutic pathways to stimulate platelet production.
As mentioned above, despite its vital role in early steps of platelet generation, the later steps, including MK maturation and platelet release, however, according to several functional and genetic studies, are TPO-independent [Citation2,Citation23,Citation27,Citation37]. Instead, a variety of pleiotropic hematopoietic growth factors other than TPO are found to be responsible for the regulation of platelet generation at different stages. For example, SCF, IL-3, IL-6 family cytokines(IL6, IL-11, and leukemia inhibitory factor) are proved to play important roles both in promoting MK proliferation and maturation, whereas GM-CSF mainly affect MK proliferation [Citation26]. In vitro studies have indicated that these factors act in a synergistic way with TPO, and some of them are capable of inducing platelet production by cultured MKs, even in the absence of TPO, probably through TPO-independent pathways [Citation26,Citation28]. These cytokines also exhibit specific changing trends in cases of thrombocytopenia. For example, many results suggested that IL-11 levels may in part be regulated by a negative feedback loop based on circulating platelet counts. Elevated IL-11 levels were observed in both hypoplasia and hyperplasia bone marrow among patients with thrombocytopenia [Citation38]. Unfortunately, there are still limited studies referring the variations of some other factors in thrombocytopenic conditions. And the relationship between the changes of these factors with the clinical progression as well as prognosis of certain diseases are still ill-defined. Further elucidations may expand the spectrum of indications of these cytokines mimetics in clinical conditions.
Chemokines
Migration of immature MKs to the vascular niche is a critical step in platelet production, as they can enter into a specific region where the environmental cues are permissive and instructive for TPO-independent MK maturation and platelet release [Citation39]. Stromal derived factor-1 (SDF-1), also named CXCL-12, is a kind of chemokine that mainly originates from bone marrow stromal cells [Citation40]. Through its specific receptor CXCR4, SDF-1 is implicated in the maturational chemotaxis of MKs toward sinusoidal vessels [Citation41]. CXCR4 is highly expressed on megakaryocytic lineage cells, which can be considered as a critical cellular signal for cell transmigration in response to gradients of SDF-1. Binding of SDF-1 to CXCR4 leads to intracellular calcium mobilization, matrix metalloproteinase 9 expression, activation of the intracellular JAK1, JAK2, STAT2, STAT4 pathway, and ultimately cell migration along the SDF-1 gradients [Citation41,Citation42]. In vitro studies have shown that SDF-1 does not induce platelet production in the absence of bone marrow endothelial cells (BMECs), whereas stabilization of endogenous SDF-1 expression or administration in vivo significantly lead to the increment of circulating platelets, accompanied by the redistribution of MKs to the vasculatures, suggesting that the direct effect of SDF-1 is to contribute to the maturational localization of MKs to the vascular niche, and the increment of thrombopoiesis is likely due to promotion effects of the vascular niche [Citation41]. Therefore, it can be concluded that the close interaction between MK and BMEC is necessary in contributing platelet production. Fibroblast growth factor-4 (FGF-4) is a kind of chemokine that can promote the adhesion of megakaryocytic cells to BMECs through upregulating the vascular cell adhesion molecule-1 (VCAM-1) expression on BMECs [Citation39]. Chemokines FGF-4 and SDF-1 both can promote thrombopoiesis through enhancing the interaction of megakaryocytic progenitors with the bone marrow vascular niche, but their exact roles are different. The SDF-1 promotes the migration of MKs to the sinusoidal vessels, while the FGF-4 enhances the adhesion of MKs to BMECs, which can all further facilitate MK maturation and thrombopoiesis [Citation39]. Thus, abnormalities of these chemokines will definitely affect the aforementioned steps in platelet production, and eventually lead to thrombocytopenia. The association of changing of these chemokines with certain thrombocytopenic diseases has yet to be elucidated. Many researchers have found the SDF-1 level and CXCR4 expression on MKs in the bone marrow were markedly decreased in children with ITP. Furthermore, the SDF-1 gene polymorphisms were closely correlated with the occurrence and prognosis of childhood ITP [Citation43,Citation44]. It has also been suggested that identification of SDF-1 single nucleotide polymorphisms in different patients may help to predict the severity of the disease and distinguish between acute and chronic ITP [Citation45]. So, further identification of the abnormalities of these progenitor-active chemokines in certain diseases is of great significance for the diagnosis, prognosis as well as treatment of these diseases.
Bioactive lipid mediator
Sphingosine-1-phosphate (S1P) is a kind of lysophospholipid that can be released from platelets, erythrocytes, mononuclear cells, and granulocytes [Citation46]. S1P mediates a variety of biological functions, such as the proliferation, migration, morphogenesis, and differentiation of cells [Citation47]. Various S1P-specific receptor have been identified, among which S1P4 is the only one that expressed in platelets and has been found to be specifically up-regulated during megakaryocytic differentiation from HSCs [Citation46,Citation48]. Studies by Golfier et al. [Citation46] have showed the importance of S1P4 in terminal MK differentiation. They observed that there was a significant increase in morphologically aberrant MKs in the bone marrow of S1P4-deficient mice, and these cells produced fewer and atypical pro-platelets in vitro. Besides, they also demonstrated that the underlying mechanism of S1P4 signaling in favoring the final differentiation steps of platelet production is likely through switching from Rho to Cdc42 activation, which may further influence the cytoskeleton activities [Citation46]. Whether or not the effect of S1P4 in contributing PPF is also true in human beings as well as the implication of defective S1P4 in the pathogenesis of certain thrombocytopenic diseases are still need to be further confirmed. Further studies will shed new light on raising S1P4 as a molecular target in clinical conditions where there is a need for a rapid increase in platelet production.
The supportive roles of none-hematopoietic cells in platelet production
Bone marrow mesenchymal stem cells
As an important ingredient that constitutes the bone marrow hematopoietic microenvironment, stromal cells also play a critical role in regulating platelet generation. There are multiple types of stromal cells within the bone marrow, among which mesenchymal stem cells (MSCs) could be considered as a flagship, especially for their capacities to promote platelet production. MSC is a kind of pluripotent stromal cell which can both self-renew as well as give rise to other types of differentiated stromal cells, such as osteolineage cells, adipocytes, chondrocytes, and endothelial cells [Citation49]. Several publications have illustrated that MSCs can sustain MK differentiation and platelet formation through both direct and indirect mechanisms. on the one hand, they can express and secret a wide range of growth factors and chemokines, including TPO, IL-6, IL-11, LIF, SCF, and SDF-1, which can mediate the indirect promoting effects of MSCs on megakaryocytic development and maturation [Citation45,Citation50]. Furthermore, early studies by Cheng et al. [Citation51] indicated that MSCs and MKs in the bone marrow have a direct contact with each other. Through co-culturing, Pallotta et al. [Citation52] also demonstrated that direct cell-cell contact, which is mediated by cell adhesion molecules and their ligands, modulates MK maturation and differentiation. They observed that co-culture of MKs with MSCs can lead to increased formation of pro-platelets, even in the absence of exogenous cytokines [Citation11]. In addition, their studies further validated the central role of integrin very late antigen-4 (VLA-4) and its ligand VCAM-1 in this promoting effects of MSCs on PPF, for inhibiting this signaling pathway with VLA-4 antagonist, PPF was significantly decreased. Further studies have suggested that the VLA-4/VCAM1 signal pathway is implicated in the rearrangements of MK cytoskeleton, so as to promote PPF [Citation53]. VACM-1 has been found to be constitutively expressed on MSCs, moreover, adhesion molecules such as intercellular adhesion molecule 1, 2 (ICAM-1, 2) and E-selectin have also been suggested to be engaged in the direct interaction between MSCs and MKs [Citation45]. Collectively, direct cell-cell interaction and cytokines secretion of MSCs simultaneously contribute to the differentiation in terminal cells of MK lineage within the bone marrow niche.
Recently, the immune regulation effects of MSCs have also been demonstrated in a large quantity of studies. MSCs exert their immune suppression effects through producing a number of molecules, including prostaglandins, IL-10, and indoleamine 2,3-dioxygenase [Citation54]. They can also induce the generation of regulatory T cells (Tregs) and inhibit functional activities of T-helper lymphocytes type-1(Th1 cells) and natural killer cells (NK cells), which can synergistically enhance their immunosuppressive effects [Citation55]. The maintenance of immunological tolerance is vital for normal platelet production, for augmented immunologic responses in the bone marrow can also influence platelet generation. The premature destruction of MKs by auto-antibodies is a common pathological mechanism of thrombocytopenia in a variety of autoimmune diseases [Citation56]. Moreover, the auto-antibodies targeting cytokines or their receptors may also significantly influence platelet generation. Researchers have found that MSCs in ITP and many other autoimmune diseases exhibited abnormal morphology, decreased proliferation and growth potential, but increased apoptosis ration [Citation6]. Dysfunctional MSCs are considered as an important underlying pathogenic mechanism in these diseases. Overall, it can be concluded that the defective MSCs found in ITP and many other autoimmune diseases may further contribute to thrombocytopenia by multiple mechanisms, so it is possible to raise MSCs as therapeutic targets for better treatment of these diseases.
Derivatives of MSCs
Some of the derivatives of MSCs in the bone marrow were also found to play critical roles in these regulation processes of platelet generation. Previous studies have indicated that opposite signals are given to HSCs by MSCs and their differentiated osteoblasts [Citation52]. Several studies have further showed that direct interaction of HSCs with osteoblasts inhibit MK maturation and PPF, which has been suggested to be correlated with type I collagen that they released [Citation52]. In contrast, adiopocytes oppose HSCs quiescence, promoting HSCs cycling and differentiation [Citation57], and thus may contribute to megakaryopoiesis to some extent [Citation52].
Bone marrow endothelial cells (BMECs) are major components of the bone marrow vascular niche. Many evidences have highlighted the significance of BMECs in regulating MK functions, in terms of differentiation, development and the release of platelets. BMECs can secret a series of chemokines and cytokines to support platelet generation, such as SCF, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β) [Citation4]. In addition, BMECs constitutively express various types of adhesion molecules, like VCAM-1 and E-selectin. Through mediating the direct contacts between MKs and BMECs, these adhesion molecules play an important role contributing to the maturation of MKs and the release of platelets [Citation53]. In a word, these endothelial factors are involved in the regulatory functions of BMECs on thrombopoiesis at different stages, which can eventually promote the production of platelets. Meanwhile, in addition to the essential roles of BMECs in supporting platelet generation, MKs were also found to have the ability to promote endothelial growth in the bone marrow [Citation11]. Some publications have demonstrated that co-culture of MKs with BMECs support the long-term survival of BMECs. Further investigations have showed that MKs can induce endothelial cell production through promoting differentiation and colony formation of endothelial progenitors [Citation58]. The interaction between MKs and BMECs can reciprocally promote each other’s growth and survival. Patients who are faced with long-term thrombocytopenia, such as ITP and congenital thrombocytopenia, often have a significant reduction in cellular elements of the vascular niche, including BMECs. Studies by Wu et al. [Citation4] showed significantly decreased cellular elements of the human bone marrow niches in patients with AA, including endosteal, vascular and perivascular cells. In consideration of the critical roles of the aforementioned cellular elements in the maintenance of megakaryopoiesis and thrombopoiesis, their deficiencies may, at least partially, pathologically exacerbate thrombocytopenia [Citation39]. Correcting this kind of defects may have important clinical implications for these patients.
The specific roles of extracellular matrix components in platelet production
The bone marrow cavity is filled with multiple types of extracellular matrix proteins, which are also suggested to provide critical signals for platelet generation. Terminal MK maturation is a process characterized by tremendous morphological changes involving rearrangements of MK membranes and cytoskeleton. This process is suggested to be closely regulated by the adhesive interactions between MKs and different types of ECM [Citation59]. Adhesion of MKs to ECM through surface integrins can activate multiple intracellular signal pathways, resulting in the changes of cytoskeleton activities. Dynamic activities of cytoskeleton proteins play the major role controlling terminal MK maturation. For example, the formation of demarcation membrane system (DMS) is of great importance during MK maturation, for it can serve as a reservoir for the membrane and organelles of pro-platelets and platelets. Previous studies have showed that the F-actin is the main driver of this DMS formation activity [Citation60]. Polymerization of tubulin in microtubule were proved to be the primary motor for pro-platelet elongation and drive the transport of organelles and granules into nascent platelets. Moreover, the actomyosin was found to be involved in pro-platelet branching and force generation during pro-platelet formation [Citation61].
The ECM, which includes fibronectin, laminin and collagen, are differentially distributed throughout the bone marrow cavity [Citation59]. Several researchers have tried to find out the distribution patterns of different types of ECM as well as their contact modes with MKs. The most accepted one has been demonstrated as that type I collagen is mainly distributed in the osteoblastic niche, whereas the vascular niche is abundant of ECM like type IV collagen, laminin and fibrinogen [Citation62]. Consistent with the functional attribute of the niche where they are located, type I collagen was found to be a negative regulator of PPF. Studies by many different groups further demonstrated that adhesion of MKs to collagen I through integrin α2β1 can activate Rho/ROCK pathway in MKs, which may promote myosin IIA phosphorylation and thus restrain MKs cytoplasmic extensions, namely inhibiting PPF [Citation62,Citation63]. This is an important mechanism to prevent premature platelet release in the bone marrow cavity. On the contrary, by counteracting collagen I-mediated inhibition, the major ECM in the vascular niche, including type IV collagen, laminin and fibrinogen can directionally support PPF at the sinusoids [Citation64]. The discrepant effects on PPF may be ascribed to peculiar structural properties of different ECM proteins and differences in receptor engagements [Citation64].
In conclusion, functional activities of these ECM proteins help to avoid ectopic platelet release and guarantee the generation of mature functional platelets that will be directly released into the peripheral blood [Citation63]. The above mentioned functional roles of ECM proteins in MK maturation are correlated with their contribution to the rearrangements of MK actin and microtublin cytoskeletons [Citation65,Citation66] Defects in any part of the ECM-receptors-cytoskeleton axis may interfere with normal MK maturation process and finally lead to thrombocytopenia. For example, several macrothrombocytopenic diseases, including the Bernard–Soulier syndrome and the May–Hegglin syndrome have been found to be related with such abnormalities [Citation23] In Bernard–Soulier syndrome, mutations of the membrane glycoprotein, a kind of ECM receptor, lead to a profound reduction in MK ability to form pro-platelet on different ECM components [Citation67] Myosin IIA is encoded by the MYH9 gene. The involvement of Myosin IIA in the inhibiting activity of type I collagen suggests a mechanism for thrombocytopenia induced by MYH9 mutations in humans, as the inability of type I collagen to repress PPF in the presence of a functionally altered myosin IIA. And this may cause the premature platelet release before MKs reach the vascular niche [Citation11,Citation64]. As mentioned above, the effects of ECM in regulating PPF are achieved mainly through their regulation of the cytoskeleton activities of MKs. Hence, we have every reason to speculate that the abnormal distribution or expression of ECM in the bone marrow niche will definitely interfere with the normal cytoskeleton activities of MKs, which will result in ectopic platelet release in the bone marrow cavity. In addition, defects in the interaction between MKs and ECM, which can be caused by abnormalities in either MK surface integrins or intracellular signal transduction proteins, or ECM proteins, will also influence the PPF process, thus leading to thrombocytopenia. Further studies are needed, so that we can find out that whether the ECM abnormalities are involved in certain diseases.
Physical microenvironment and sympathetic modulation
It has been suggested that the compositions of bone marrow niches and their interactions with hematopoietic cells are coordinated by hormonal signals, oxygen tension, and likely other physiologic stimuli. Researches over the past few decades have demonstrated that reactive oxygen species (ROS) play an essential role in certain cellular events. Through promoting intracellular ROS generation, the important role of oxidative stress in promoting MK development, including differentiation, maturation, polyploidization, and pro-platelet fragmentation, have also recently been highlighted [Citation68]. It is well known that the distribution of HSCs in the bone marrow is closely associated with O2 tension, and the lowest O2 level in the osteoblastic niche help to prevent CD34+ HSCs from entering into the cell cycle and maintain them in a quiescent state for self-renewal [Citation69]. Differentiation of HSCs toward definite lineages are controlled by specific extracellular or intracellular agonists. As mentioned above, TPO is a kind of extracellular agonist that can drive HSCs to differentiate into the megakaryocytic lineage. Recently, ROS has also been indicated as an important microenvironmental factor that acts on the differentiation phase of HSCs into MKs, as increased total number of MKs and CFU-MKs from CD34+ cells were observed when in a more oxygenated environment [Citation70]. In addition, ROS is also participate in MK proliferation and differentiation, for MK often show a greater size and higher ploidy under higher O2 tension conditions. When MKs migrate to the vascular niche, the increments in either O2 tension or ROS promotes the maturation of MKs and the subsequent PPF step [Citation70]. Although the details of the mechanism of ROS in regulating cellular signaling pathways during megakaryocytic development have not been fully uncovered, it is clear that the gradual rise in ROS levels can definitely contribute to the MK development. Therefore, increasing the level of ROS either through the enhancement of ROS generation or inhibition of intracellular antioxidants will provide potential therapeutic avenues for rescuing thrombocytopenia [Citation10].
Previous studies have demonstrated substantial sympathetic innervation in the bone marrow, and the transmitters released from sympathetic nerve terminals, including norepinephrine (NE) and epinephrine (EPI), are effective in increasing the number of CD34+ cells [Citation71]. Recently, some in vitro studies also revealed that in addition to the expansion of CD34+ cells, both NE and EPI can stimulate platelet production through promoting MK adhesion, migration, and PPF via α2-adrenoceptor–mediated extracellular signal-regulated kinase (ERK) activation [Citation72]. Results from all these studies indicate that in addition to the substantial components, physical and neural regulation also influence platelet generation, which may also be applied to increasing platelet generation in certain clinical conditions.
Conclusions
Platelets play irreplaceable roles in daily physiological activities. Many efforts have been directed to define the mechanisms underlying an efficient platelet production, so as to develop effective therapies that can be applied in clinical conditions of thrombocytopenia. As mentioned above, platelet generation in the bone marrow can be divided into two main stages, and each stage is finely orchestrated by specific niche factors (as diagrammatic presented in ). In this review, we discussed the functional roles of each ingredient of BMM in platelet production, respectively. In fact, platelet generation is a continuous process, and the regulatory activities of these microenvironmental factors are not entirely separate. For example, stromal cells rely on their secreted cytokines or adhesion molecules to play their regulatory roles. Specific cells like MSCs and BMECs may exert their promoting effects through multiple mechanisms. The coordinated work of these components guarantee the whole differentiation process to proceed in an orderly way. Meanwhile, abnormal expression of any of these components will definitely influence platelet generation through affecting one or more steps. Not surprisingly, recent studies have found a variety of thrombocytopenic diseases to be related with abnormalities of microenvironment regulators. And this has driven the developments of a series of therapies targeting these abnormalities. For example, in patients with decreased production or increased destruction of some growth factors, we can supply them with exogenous growth factors mimics or receptor agonists. Similarly, immunoregulatory therapies can be used when patients have immunity disorders. However, what we have known is still very limited and what kinds of abnormalities are involved in certain thrombocytopenic diseases are still need to be further explored. Furthermore, treatments that only target microenvironmental factors are not always satisfactory. Considering that these microenvironmental factors still need to regulate a series of intracellular reactions to exert their effects, researches involving the intracellular signaling pathways and intracellular regulatory factors are of great importance in our future studies. In addition, some cell intrinsic defects may also have serious impacts on platelets generation. Identification of the variations of both the niche ingredients and their downstream factors will be helpful for developing more effective therapeutic approaches. Moreover, both the niche compositions and their interactions with hematopoietic cells are coordinated by hormonal signals, oxygen tension, and some other physiologic stimuli, which can also be applied to increase platelet production in some conditions. In summary, future studies should focus on finding out alternations of these niche components as well as their downstream regulatory factors in certain thrombocytopenic diseases, and how these factors can be exploited to improve treatments as well as prognosis of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Xu XR, Zhang D, Oswald BE, et al. Platelets are versatile cells: new discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53(6):409–430. doi: 10.1080/10408363.2016.1200008
- Bianchi E, Norfo R, Pennucci V, et al. Genomic landscape of megakaryopoiesis and platelet function defects. Blood. 2016;127(10):1249–1259. doi: 10.1182/blood-2015-07-607952
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984
- Wu L, Mo W, Zhang Y, et al. Impairment of hematopoietic stem cell niches in patients with aplastic anemia. Int J Hematol. 2015;102(6):645–653. doi: 10.1007/s12185-015-1881-2
- Balderman SR, Calvi LM. Biology of BM failure syndromes: role of microenvironment and niches. Hematol Am Soc Hematol Educ Program. 2014;2014(1):71–76.
- Zhang D, Li H, Ma L, et al. The defective bone marrow-derived mesenchymal stem cells in patients with chronic immune thrombocytopenia. Autoimmunity. 2014;47(8):519–529. doi: 10.3109/08916934.2014.938320
- Woolthuis CM, Park CY. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood. 2016;127(10):1242–1248. doi: 10.1182/blood-2015-07-607945
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007
- Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041
- Chen S, Su Y, Wang J. ROS-mediated platelet generation: a microenvironment-dependent manner for megakaryocyte proliferation, differentiation: and maturation. Cell Death Dis. 2013;4:e722. doi: 10.1038/cddis.2013.253
- Malara A, Abbonante V, Di Buduo CA, et al. The secret life of a megakaryocyte: emerging roles in bone marrow homeostasis control. Cell Mol Life Sci. 2015;72(8):1517–1536. doi: 10.1007/s00018-014-1813-y
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25.
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040
- de Barros AP, Takiya CM, Garzoni LR, et al. Osteoblasts and bone marrow mesenchymal stromal cells control hematopoietic stem cell migration and proliferation in 3D in vitro model. PLoS One. 2010;5(2):e9093. doi: 10.1371/journal.pone.0009093
- Ema H, Suda T. Two anatomically distinct niches regulate stem cell activity. Blood. 2012;120(11):2174–2181. doi: 10.1182/blood-2012-04-424507
- Eto K, Kunishima S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood. 2016;127(10):1234–1241. doi: 10.1182/blood-2015-07-607903
- May G, Enver T. Lineage specification: reading the instructions may help! Curr Biol. 2013;23(15):R662–R665. doi: 10.1016/j.cub.2013.06.054
- Endele M, Etzrodt M, Schroeder T. Instruction of hematopoietic lineage choice by cytokine signaling. Exp Cell Res. 2014;329(2):207–213. doi: 10.1016/j.yexcr.2014.07.011
- Sim X, Poncz M, Gadue P, et al. Understanding platelet generation from megakaryocytes: implications for in vitro-derived platelets. Blood. 2016;127(10):1227–1233. doi: 10.1182/blood-2015-08-607929
- Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol. 2014;165(2):259–268. doi: 10.1111/bjh.12772
- Deutsch VR, Tomer A. Megakaryocyte development and platelet production. Br J Haematol. 2006;134(5):453–466. doi: 10.1111/j.1365-2141.2006.06215.x
- Chang Y, Aurade F, Larbret F, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109(10):4229–4236. doi: 10.1182/blood-2006-04-020024
- Morishima N, Nakanishi K. Proplatelet formation in megakaryocytes is associated with endoplasmic reticulum stress. Genes Cells. 2016;21(7):798–806. doi: 10.1111/gtc.12384
- Sasaki H, Hirabayashi Y, Ishibashi T, et al. Effects of erythropoietin, IL-3, IL-6 and LIF on a murine megakaryoblastic cell line: growth enhancement and expression of receptor mRNAs. Leuk Res. 1995;19(2):95–102. doi: 10.1016/0145-2126(94)00121-P
- Zheng C, Yang R, Han Z, et al. TPO-independent megakaryocytopoiesis. Crit Rev Oncol Hematol. 2008;65(3):212–222. doi: 10.1016/j.critrevonc.2007.11.003
- Ng AP, Kauppi M, Metcalf D, et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci USA. 2014;111(16):5884–5889. doi: 10.1073/pnas.1404354111
- Tong W, Lodish HF. Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J Exp Med. 2004;200(5):569–580. doi: 10.1084/jem.20040762
- Methia N, Louache F, Vainchenker W, et al. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood. 1993;82(5):1395–1401.
- Choi ES, Nichol JL, Hokom MM, et al. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85(2):402–413.
- Kaushansky K, Broudy VC, Lin N, et al. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci USA. 1995;92(8):3234–3238. doi: 10.1073/pnas.92.8.3234
- Kuwana M, Okazaki Y, Kajihara M, et al. Autoantibody to c-Mpl (thrombopoietin receptor) in systemic lupus erythematosus: relationship to thrombocytopenia with megakaryocytic hypoplasia. Arthritis Rheum. 2002;46(8):2148–2159. doi: 10.1002/art.10420
- Fielder PJ, Hass P, Nagel M, et al. Human platelets as a model for the binding and degradation of thrombopoietin. Blood. 1997;89(8):2782–2788.
- Emmons RV, Reid DM, Cohen RL, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87(10):4068–4071.
- Makar RS, Zhukov OS, Sahud MA, et al. Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol. 2013;88(12):1041–1044. doi: 10.1002/ajh.23562
- Zhu GR, Zhou XY, Lu H, et al. Human bone marrow mesenchymal stem cells express multiple hematopoietic growth factors. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11(2):115–119.
- Paulus JM, Debili N, Larbret F, et al. Thrombopoietin responsiveness reflects the number of doublings undergone by megakaryocyte progenitors. Blood. 2004;104(8):2291–2298. doi: 10.1182/blood-2003-05-1745
- Chang M, Suen Y, Meng G, et al. Differential mechanisms in the regulation of endogenous levels of thrombopoietin and interleukin-11 during thrombocytopenia: insight into the regulation of platelet production. Blood. 1996;88(9):3354–3362.
- Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10(1):64–71. doi: 10.1038/nm973
- Feng Y, Chen X, Gao L. Hypothesis: human umbilical cord blood-derived stromal cells promote megakaryocytopoiesis through the influence of SDF-1 and PECAM-1. Cell Biochem Biophys. 2010;58(1):25–30. doi: 10.1007/s12013-010-9086-z
- Niswander LM, Fegan KH, Kingsley PD, et al. SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood. 2014;124(2):277–286. doi: 10.1182/blood-2014-01-547638
- Lane WJ, Dias S, Hattori K, et al. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood. 2000;96(13):4152–4159.
- Ku FC, Tsai CR, Der Wang J, et al. Stromal-derived factor-1 gene variations in pediatric patients with primary immune thrombocytopenia. Eur J Haematol. 2013;90(1):25–30. doi: 10.1111/ejh.12025
- Sheng GY, Huang XL, Bai ST. Expression levels of CXCR4 on megakaryocytes and its ligand in bone marrow in children with acute idiopathic thrombocytopenic purpura. Zhonghua Er Ke Za Zhi. 2004;42(7):499–501.
- Khodadi E, Asnafi AA, Shahrabi S, et al. Bone marrow niche in immune thrombocytopenia: a focus on megakaryopoiesis. Ann Hematol. 2016;95(11):1765–1776. doi: 10.1007/s00277-016-2703-1
- Golfier S, Kondo S, Schulze T, et al. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010;24(12):4701–4710. doi: 10.1096/fj.09-141473
- Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31(Pt 6):1216–1219. doi: 10.1042/bst0311216
- Motohashi K, Shibata S, Ozaki Y, et al. Identification of lysophospholipid receptors in human platelets: the relation of two agonists, lysophosphatidic acid and sphingosine 1-phosphate. FEBS Lett. 2000;468(2–3):189–193. doi: 10.1016/S0014-5793(00)01222-9
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143
- Garcia-Garcia A, de Castillejo CL, Mendez-Ferrer S. BMSCs and hematopoiesis. Immunol Lett. 2015;168(2):129–135. doi: 10.1016/j.imlet.2015.06.020
- Cheng L, Qasba P, Vanguri P, et al. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34(+) hematopoietic progenitor cells. J Cell Physiol. 2000;184(1):58–69. doi: 10.1002/(SICI)1097-4652(200007)184:1<58::AID-JCP6>3.0.CO;2-B
- Pallotta I, Lovett M, Rice W, et al. Bone marrow osteoblastic niche: a new model to study physiological regulation of megakaryopoiesis. PLoS One. 2009;4(12):e8359. doi: 10.1371/journal.pone.0008359
- Schweitzer KM, Drager AM, van der Valk P, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148(1):165–175.
- Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36(10):2566–2573. doi: 10.1002/eji.200636416
- Ma S, Xie N, Li W, et al. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21(2):216–225. doi: 10.1038/cdd.2013.158
- Stasi R. Immune thrombocytopenia: pathophysiologic and clinical update. Semin Thromb Hemost. 2012;38(5):454–462. doi: 10.1055/s-0032-1305780
- Seshadri M, Qu CK. Microenvironmental regulation of hematopoietic stem cells and its implications in leukemogenesis. Curr Opin Hematol. 2016;23(4):339–345. doi: 10.1097/MOH.0000000000000251
- Kwon SM, Lee JH, Lee SH, et al. Cross talk with hematopoietic cells regulates the endothelial progenitor cell differentiation of CD34 positive cells. PLoS One. 2014;9(8):e106310. doi: 10.1371/journal.pone.0106310
- Klamer S, Voermans C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh Migr. 2014;8(6):563–577. doi: 10.4161/19336918.2014.968501
- Antkowiak A, Viaud J, Severin S, et al. Cdc42-dependent F-actin dynamics drive structuration of the demarcation membrane system in megakaryocytes. J Thromb Haemost. 2016;14(6):1268–1284. doi: 10.1111/jth.13318
- Malara A, Balduini A. Blood platelet production and morphology. Thromb Res. 2012;129(3):241–244. doi: 10.1016/j.thromres.2011.11.042
- Sabri S, Jandrot-Perrus M, Bertoglio J, et al. Differential regulation of actin stress fiber assembly and proplatelet formation by alpha2beta1 integrin and GPVI in human megakaryocytes. Blood. 2004;104(10):3117–3125. doi: 10.1182/blood-2003-12-4398
- Semeniak D, Kulawig R, Stegner D, et al. Proplatelet formation is selectively inhibited by collagen type I through Syk-independent GPVI signaling. J Cell Sci. 2016;129(18):3473–3484. doi: 10.1242/jcs.187971
- Balduini A, Pallotta I, Malara A, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6(11):1900–1907. doi: 10.1111/j.1538-7836.2008.03132.x
- Gobbi G, Mirandola P, Carubbi C, et al. Proplatelet generation in the mouse requires PKCepsilon-dependent RhoA inhibition. Blood. 2013;122(7):1305–1311. doi: 10.1182/blood-2013-04-490599
- Pleines I, Dutting S, Cherpokova D, et al. Defective tubulin organization and proplatelet formation in murine megakaryocytes lacking Rac1 and Cdc42. Blood. 2013;122(18):3178–3187. doi: 10.1182/blood-2013-03-487942
- Balduini A, Malara A, Balduini CL, et al. Megakaryocytes derived from patients with the classical form of Bernard-Soulier syndrome show no ability to extend proplatelets in vitro. Platelets. 2011;22(4):308–311. doi: 10.3109/09537104.2010.547960
- Eliades A, Matsuura S, Ravid K. Oxidases and reactive oxygen species during hematopoiesis: a focus on megakaryocytes. J Cell Physiol. 2012;227(10):3355–3362. doi: 10.1002/jcp.24071
- Hermitte F, Brunet de la Grange P, Belloc F, et al. Very low O2 concentration (0.1%) favors G0 return of dividing CD34+ cells. Stem Cells. 2006;24(1):65–73. doi: 10.1634/stemcells.2004-0351
- Mostafa SS, Miller WM, Papoutsakis ET. Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. Br J Haematol. 2000;111(3):879–889.
- Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8(10):1123–1131. doi: 10.1038/ni1509
- Chen S, Du C, Shen M, et al. Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood. 2016;127(8):1024–1035. doi: 10.1182/blood-2015-07-660746