ABSTRACT
Objective: Relapse is the major cause of treatment failure in acute lymphoblastic leukemia (ALL) of childhood; it is more frequent among high-risk patients from low-middle income than from high-income countries. The frequency, sites and outcome of relapsed ALL in children of northeast Mexico over a decade was documented.
Methods: A retrospective analysis of 246 children belonging to a low-income group <16 years with de novo ALL during 2004–2015 was performed. Five-year overall survival (OS) and event-free survival was estimated by Kaplan–Meier analysis. Data on time, site, response to therapy and final outcome of relapse were analyzed. Hazard ratios (HRs) of relapse and death were estimated by the Cox regression model. Very early relapse was defined as that occurring in <18 months, early relapse between 18 and 36 months, and late relapse >36 months from diagnosis, respectively.
Results: Eighty-seven (35.4%) children relapsed. Five-year OS was 82.6% in children without relapse vs. 42% for relapsed patients. Bone marrow (BM) was the most frequent site of relapse (51.72%). Isolated central nervous system (CNS) relapses occurred in 29.9%. Five-year OS was 11.2% for BM and 15.5% for early relapse. HR of relapse for organomegaly was 3.683, 2.247 for an initial white blood cell count >50 000 × 109/l and 1.169 for positive minimal residual disease status.
Conclusion: A high rate of very early, CNS, and BM relapse with a considerably low 5-year OS requiring reassessment of therapy was documented. Organomegaly at diagnosis was a highly significant clinical predictor for relapse.
Introduction
Acute lymphoblastic leukemia (ALL) accounts for 25% of cancers before 15 years of age [Citation1]. In the United States, the incidence is 30 cases per million persons younger than 20 years [Citation2]. Incidence of ALL in Mexico and for Hispanic populations in the United States is 40 cases per million [Citation3]. In the United States, the overall survival (OS) with risk-directed treatment is 90% [Citation1]. Cure rates are inferior in low-income countries, where the majority of children with ALL live [Citation4].
The intensity of treatment in ALL is based on risk of relapse, predicted by a combination of clinical, cytogenetic, and morphological response criteria [Citation5]. However, risk groups identified by these variables are fairly nonspecific because a great number of relapses occur in the standard-risk group [Citation6]. Risk stratification is further improved by cytogenetics and minimal residual disease (MRD) studies. Although MRD is the most sensitive and specific predictor of relapse risk [Citation7,Citation8], it is not always available in low- or middle-income countries.
Relapse is the major cause of treatment failure in 15–20% of patients, with long-term OS around 36% [Citation9]. intensity of contemporary ALL chemotherapy protocols prevent further intensification in relapsed children, as the risk of toxic death increases substantially and even with hematopoietic stem cell transplant the probability of curing the disease is about 25% [Citation10].
The majority of relapses in ALL occur in the bone marrow (BM) alone or combined, mainly with the central nervous system (CNS) or testes [Citation11]. Survival can be predicted by sites involved at relapse, length of first complete remission, time from diagnosis to relapse, and immunophenotype of relapsed ALL [Citation12]. Only 30–50% of relapsed ALL children can be cured in high-income countries. In low-middle-income nations, few reports of relapse rates and its clinical outcome exist [Citation13,Citation14]. We report our findings over a 10-year period involving children diagnosed with ALL attending a reference hematology center in Latin America and suffering a relapse.
Methods
This observational, longitudinal, and retrospective study included 246 patients younger than 16 years with newly diagnosed ALL from 2004 to 2015 at the Hematology Department of the Dr José E. González University Hospital of the School of Medicine of the Universidad Autónoma de Nuevo León in Monterrey, México who received treatment at the institution and had complete information available. This is an academic reference center for low-income uninsured patients in the northeast region of the country. The study protocol was approved by the Institutional Research Board and Ethics Committee.
Diagnosis
The diagnosis, immunophenotype, and detection of MRD of ALL were confirmed by multiparametric flow cytometry. Complete remission was defined as the presence of less than 5% lymphoblasts in the BM aspirate, a neutrophil count ≥1.0 × 109/l and a platelet count ≥100 × 109/l in peripheral blood and morphologically as <5% blasts in the BM accompanied by normal hematopoiesis. MRD detection by flow cytometry was considered to be present in patients if >0.01% [Citation15], patients with MRD levels ≥0.01% were assigned into the high-risk group and were treated accordingly with a more intensive chemotherapy regimen. CNS involvement was diagnosed if leucocytes were present in cerebrospinal fluid (CSF) and lymphoblasts were identified unequivocally in cytospin preparations and CSF flow cytometry [Citation16]. For treatment purposes, patients were not assigned to CNS 2 or 3 categories, instead all children with lymphoblasts in CSF were treated with a regimen based on a previous report by the Pediatric Oncology Group for isolated CNS relapse [Citation17].
Those patients younger than 1 year and older than 10, with >50 × 109/l white blood cells (WBCs), infiltration of the CNS and/or testis at presentation, T-cell ALL, CD10-negative antigen, poor response to steroids, or with a lack of remission after 29 days of starting induction therapy, were considered to be at high risk and were treated accordingly [Citation6,Citation18]. Organomegaly and lymphadenopathy was documented by clinical examination.
Beginning in 2009, flow cytometry MRD studies were performed at the end of induction on day 29 in 126 of included patients. Owing to financial restrictions, cytogenetic studies were not performed. Response to steroids was determined using the absolute count of blasts in the peripheral blood on day 8 after 7 days of steroid administration.
Relapse
Relapse was defined as re-emergence of the disease following known criteria for BM, combined [Citation19] and for extramedullary relapses [Citation20]. Relapse occurring <18 months after diagnosis was defined as a very early relapse, a relapse occurring between 18 and 36 months was designed as an early relapse and that developing >36 months from diagnosis was classified as late relapse [Citation19].
Treatment
Children were stratified into standard and high-risk groups according to National Cancer Institute (NCI) Rome risk criteria to receive risk-adapted chemotherapy [Citation21]. Between January 2004 and April 2009 patients received a protocol for children with a new diagnosis of ALL designed at our center based on drug availability and financial considerations in our uninsured population; induction to remission included standard doses of prednisone (PDN), vincristine (VCR), l-asparaginase (l-ASP), and one or two doses of doxorubicin (DOX) in standard and high-risk children, respectively, plus four doses of triple intrathecal chemotherapy for CNS prophylaxis. Briefly, consolidation included single doses of cytosine arabinoside (Ara-C), 1.5 g/m2, and methotrexate (MTX), 1.5 g/m2 administered in a 24-h infusion. Folinic acid, 10–15 mg/m2 orally was administered 24 h after infusion of MTX ended. Afterwards children received a month of daily 6 mercaptopurine (6MP) and weekly MTX. Intrathecal chemotherapy was administered during consolidation on days 36 and 43. Reinduction consisted in 15 days of PDN, three doses of VCR, two of DOX for high and one for standard-risk patients, two doses of l-ASP and two of triple intrathecal CNS prophylaxis. Ten days after reinduction, maintenance was started for 90 weeks and included daily doses of 6MP and MTX weekly. Every 6 weeks during the first year of maintenance, and every 3 months for three times a year during the second year, maintenance was suspended for a week to administrate one single of VCR, TIC, and 7 days of PDN [Citation22].
After relapse, second line therapy consisted of VCR 1.5 mg/m2 and daunorubicin 25 mg/m2 per week for 4 weeks; PDN 60 mg/m2 per day for 28 days; l-ASP 6000 units/m2 i.m. thrice weekly for nine doses and intrathecal chemotherapy on days 0, 7 and 28. If the patient reached second CR, post-induction intensification followed.
After April 2009, to decrease therapy-related death and toxicity requiring hospitalization, treatment changed to one in which administration of high systemic doses of MTX and Ara-C was omitted, as recently reported [Citation22]. Overall duration of treatment was 30 months for girls and 36 months for boys.
In patients who relapsed, reinduction therapy was administered, it consisted of VCR 1.5 mg/m2 and daunorubicin 25 mg/m2 per week for 4 weeks; PDN 60 mg/m2 per day for 28 days; l-ASP 6000 units/m2 i.m. thrice weekly for nine doses and intrathecal chemotherapy on days 0, 7 and 28. After CR, post-induction intensification was administered.
In both treatment protocols, craniospinal irradiation was given to all patients with isolated and combined CNS relapse. These patients received 18 Gy to the brain divided in nine daily sessions. Intrathecal hydrocortisone was administered following radiotherapy. Patients with CNS infiltration at diagnosis or CNS relapse were treated with the aforementioned scheme for isolated CNS relapse [Citation17], irrespective of the CSF blast count.
Patients who abandoned treatment during the study period were excluded from analysis.
Statistical analysis
We conducted a detailed review of the clinical and electronic records of 246 patients diagnosed with ALL from January 2004 to June 2015 and built a database using SPSS version 20 statistical package. Patients were grouped per risk at diagnosis and by the time and site of relapse. Clinical and biological features of children suffering a relapse were compared to non-relapsed patients using a univariate analysis with the X2 test for categorical variables. Event-free survival (EFS) was defined from the chemotherapy starting date until the date of non-response to therapy, abandonment of treatment, relapse, or death from any cause; relapse or the last follow-up [Citation23]. OS was calculated from the chemotherapy starting date until the date of death or the last follow-up [Citation11]. OS and EFS were calculated according to the Kaplan–Meier method [Citation24] and groups were compared by the log-rank test [Citation25]. Cox proportional-hazard regression model [Citation26] was used for uni- and multivariate analysis of prognostic factors with 95% confidence intervals. In addition to known risk variables, adenopathy and organomegaly were included in the Cox regression model for risk of relapse and death to assess its impact in ALL course. A p-value of <0.05 was considered statistically significant.
Results
During the study period 273 children were diagnosed with ALL, 14 (5%) abandoned treatment and 13 (4.75%) received chemotherapy at other institutions. The presenting clinical characteristics of the 246 patients included in this report are shown in , whereas patient distribution, risk group, relapse site and time of relapse are shown in . Median age at diagnosis was 5 years (0–15); over half of children were 1–5 years old (51.2%).
Figure 1. Consort diagram showing relapse percentage, sites and time of relapse for 246 children with ALL. For clarity, one patient who relapsed to the testis is not shown. Fourteen patients (5%) abandoned treatment and 13 (4.8%) received chemotherapy at other institutions.
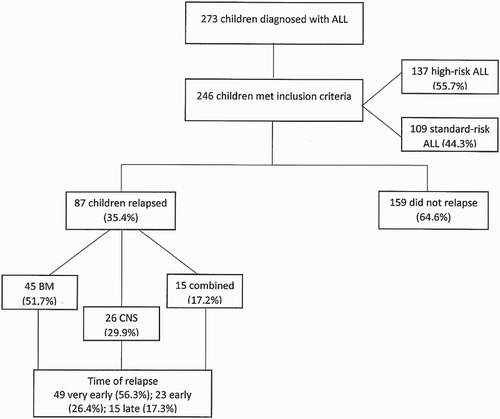
Table 1. Characteristics of the 246 children with ALL included in the study.
Overall outcome of children with ALL
Two-hundred and ten (90.5%) children achieved complete remission (CR) and 15 (6%) died during induction. Seven deaths during induction occurred among 51 patients with an initial WBC count ≥50 000 vs. 8 deaths from 194 patients with ≤50 000 WBC count (p = 0.011). Five of 91 patients with organomegaly at diagnosis died during induction vs. 10/153 from those without visceromegalies (p = 0.753). Adenopathies were present in 58 patients at diagnosis, 2 of them died during induction vs. 8/194 with no adenopathies (p = 0.331).
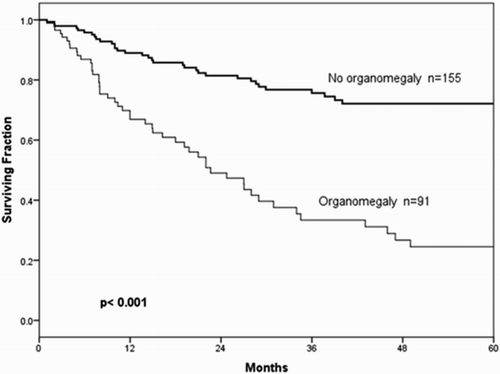
EFS at 5 years was 54.9 ± 4.09%. Median EFS at 5 years was not reached. After 5 years of follow-up, OS was 64.0 ± 4.11%, median OS was not reached. Males had a significant higher relapse rate (62.1%) than females (37.9%) and their 5-year EFS was 48.95 vs. 62.2%, respectively, (p = 0.008). EFS at 5 years in high-risk children was 42.9 ± 5.61% with a median of 40.01 months vs. 69.3 ± 5.24% in the standard-risk group (p = 0.001), not shown. OS at 5 years in the high-risk group was 51 ± 5.51%, with a median of 60.91 months vs. 80.9 ± 4.35% in the standard-risk group (p < 0.001). Neither median OS nor EFS was reached in the standard-risk group. Five-year EFS in patients with organomegaly at diagnosis was 24.5 ± 5.87% vs. 72.1 ± 4.55% for those without (p < 0.001) (). Median EFS for patients with organomegaly at diagnosis was 22.63 months and for patients without organomegaly, the median was not reached.
Figure 2. EFS at 5 years in 246 children newly diagnosed with ALL without organomegaly at diagnosis was 72.1% (95% CI 72.02–72.18) vs. 24.5% (95% CI 24.39–24.61) in those with organomegaly, p < 0.001.
MRD was determined in 126 patients after 2009; 48 (38.1%) were positive and 78 (61.9%) negative. MRD was documented in a higher percentage of high-risk patients, 30/63 (47.6%) vs. 18/63 (28.6%) in the standard-risk group, (p = 0.028). Five-year EFS for the 48 children with an MRD-positive after induction to remission (IR) therapy was 35.0 ± 3.94% at a median of 34.5 months vs. 56.2 ± 3.37% in those with an MRD-negative result (p = 0.561).
Seventy-five deaths (30.5%) were documented in the whole group and the most frequent cause was sepsis, in thirty-two patients (42.67%), . Fifty-one (68%) deaths occurred in relapsed patients at a median 47.24 months vs. 24 deaths (32%) reported from non-relapsed patients or deaths during induction, in whom median OS was not reached (p < 0.001). Of nine deaths during CR five were due to infectious complications leading to septic shock, and one each due to pulmonary edema, cardiac insufficiency, gastrointestinal bleeding, and leukoencephalopathy. Regarding risk group, 59 (78.7%) deaths occurred in high risk and 16 (21.3%) in standard-risk children (p < 0.001).
Table 2. Time and causes of death for 75 children with ALL.
EFS did not differ statistically between chemotherapy regimens (p = 0.088).
Predictive factors of relapse and death
We examined interactions among conventional presenting clinical and laboratory features at initial diagnosis with risk of relapse and death. The results of Cox proportional-hazard model are presented in . Univariate analysis showed that age (p = 0.038), male gender (p = 0.009), WBC > 50 000 (p < 0.001), T-cell ALL (p = 0.013), organomegaly (p < 0.001) and high risk at diagnosis (p < 0.001) had a significant impact on EFS. In multivariate Cox regression analysis, only organomegaly was statistically significant for EFS and it correlated with a three-time increased risk of relapse (p = 0.014). Similar results were documented in univariate analysis for OS. Age < 1 and >10 (p = 0.007), WBC count >50 000 (p = 0.001), T-cell ALL (p < 0.001), CD10-negative antigen (p < 0.001), high-risk disease (p < 0.001) and organomegaly at diagnosis (p = 0.017) were all significant predictors for lower survival. Also, poor response to steroids (p = 0.001) 8 days after starting induction to remission therapy was associated with a worst prognosis. In multivariate analysis, fulfilling the criteria for high-risk assignation significantly correlated with increased risk of death (p = 0.018).
Table 3. HR for death (s OS) and treatment failure (-f EFS) according to uni- and multivariate Cox’s proportional regression analysis for ALL in children and its association with clinical and laboratory characteristics.
Relapsed patients had more than four times the risk of death than patients without relapse (p < 0.001). Very early BM relapse (p < 0.001) was a significant predictor for lower survival with a four times greater risk of death.
With exception of 15 patients that died during induction to remission and 12 not complying, all remaining 219 children received and completed full intended treatment. Compliance with maintenance therapy was assessed during monthly follow-up visits asking face-to-face if drug doses were omitted in two or more occasions without medical indication, and this event was documented in the clinical and electronic files. Twelve children had poor compliance during maintenance; differences between these children and those who were adherent to chemotherapy were found. Relapse occurred more frequently in patients with poor adherence than in those who complied, 8/12 (66.7%) vs. 79/219 (36.07%), respectively, (p = 0.038). Death occurred in a higher percentage of patients with poor adherence, 7/12 (58.3%) vs. 54/219 (24.65%) of those fully adherent, (p = 0.010).
Outcome in relapsed patients
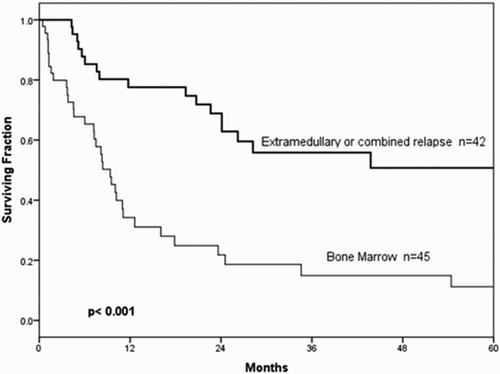
Of the 246 patients studied, 87 (35.4%) relapsed. Very early relapse was documented in 49 (56.3%) children, early in 23 (26.4%), and late relapse in 15 (17.3%). Of the 49 very early relapsed patients, 38 (64.4%) were initially stratified as high risk (p = 0.027). Most relapses, 45 (51.7%), occurred exclusively in BM, whereas 26 (29.9%) relapsed exclusively in the CNS, 1 (1.1%) in the testes and 15 (17.3%) had combined BM, testicle and CNS relapse. A statistically significant difference between BM and other sites of relapse was demonstrated (p < 0.001) ().
Outcome by time from diagnosis and anatomic site of relapse
Of the 46 BM relapsed children, 25 (54.3%) were classified as very early, 13 (28.3%) as early, and 8 (17.4%) as late relapses. CNS relapse was documented in 26 patients, 18 (69.2%) were very early, 4 (15.4%) early and 4 (15.4%) late relapses. From 21 (8.5%) patients with CNS disease at diagnosis (), 4 (19%) relapsed exclusively to the CNS at a median of 11 months; none of these four patients suffered a second CNS relapse.
Discussion
Relapse of ALL is a catastrophic event that leads to greatly reduced chances of surviving, and it is the most common cause of treatment failure. Relapse occurred in 87 (35.4%) of 246 children diagnosed and treated in our center during the study period, twice the number compared to recent reports in high-income countries where the relapse rate is 15–20% [Citation27,Citation28]. The frequency was also higher than that reported in a study from Mexico City, which found a relapse rate of 26.2% among 302 recruited patients [Citation13]. In other studies reported by Latin American countries, like Argentina between1996 and 2012, a 22% of cumulative incidence of BM relapse was found, with isolated CNS relapse less than 10% [Citation29], whereas in 444 Chilean patients relapse rate according to risk classification at diagnosis was 16–24%, with isolated CNS relapse in 5.4% children [Citation30]. In this last study, a statistically significant difference according to the use or not of high doses of MTX as a risk factor for relapse was found.
Most of our patients belonged to the high-risk group, something that was significantly reflected in the high rate of relapse encountered. A poorer prognosis of ALL is recognized in boys [Citation31]. This sex-related difference is independent from other prognostic factors; the reasons, however, are not clear or fully elucidated, but it has been suggested that genetic and epigenetic characteristics play a role in response of leukemic cells to therapy [Citation32]. In addition to the pressing need for intensified dose-dense chemotherapy protocols to decrease relapse rate in populations similar to ours, the higher risk of ALL relapse in Hispanic children is at least partly attributable to genomic variations. Then molecular genetics studies to identify specific mutations, deletions and polymorphisms in the Hispanic population allowing modification of international treatment protocols to more precisely target identified abnormalities and assign risk group in our population are required.
From these results, it is evident that for standard- and high-risk children alike, treatment intensity differentiation was inadequate in both protocols, thus a reappraisal of therapeutic schemes is ongoing at our center and moderate-high doses of MTX and Ara-C have been reintroduced in current protocols.
Remarkably, T-cell ALL incidence was under 5% in our group, this is less than half the 12.4% found in children from Mexico City [Citation3] and also lower than 10% in Chilean patients [Citation30]; this could be partially be explained by geographical variation in ALL biologic subtype distribution, as our ethnic composition in considerable different from the center and south regions of the country.
Nutritional status has been variably associated to risk of relapse [Citation33], we did not incorporate nutrition status analysis in this report, however, we have previously demonstrated in this population that both, nutrition status and body composition as assessed by dual energy X-ray absorptiometry at diagnosis of ALL is within normal parameters [Citation34].
A remarkable finding in our study was that approximately 30% of relapses were confined exclusively to the CNS. Isolated CNS relapse, reported by the Dutch Childhood Oncology Group, was 16.54% for the whole group of relapsed patients treated with their protocol ALL 9 [Citation28], almost half of our results. Similarly, in the Intercontinental trial ALL- Intercontinental Berlin-Frankfurt-Münster 2002, the percentage of patients with an ICNS was 10%, a third of our results [Citation35]. This finding was not explained by administration of intrathecal methotrexate (ITM) as a single agent in CNS prophylaxis for 129 children treated at our center, in comparison to triple intrathecal therapy (TIT) administered to 117 patients of our 26 isolated CNS relapsed patients, 13/117 were treated with TIT vs. 13/129 with single ITM (p = 0.792). In contrast, results of a trial from the Childreńs Cancer Group (CCG) reported lower incidence of isolated CNS relapse among patients treated with TIT than with ITM (p = 0.004) [Citation36]. A factor favoring CNS relapse in our group could be the timing for first lumbar puncture, which was performed around day 3. Our unusual high rate of CNS relapse can most probably be explained by poor control of marrow disease due to omission of high systemic doses of MTX, thus considerably decreasing systemic treatment intensity. A delay in establishing the diagnosis of ALL due to late referral could also have favored relapse.
Children with very early relapse in BM had a significant worse OS than those with late relapse in this same site, as reported by other researchers [Citation37]. Then, our BM relapsed patients had a poor OS when compared with other groups; for example, in a study from Finland 5-year OS for BM relapsed patients was 37% vs. 11.2% in our study [Citation9].
Interestingly, patients with hepatomegaly and/or splenomegaly had almost four times the risk for relapse in univariate analysis and this remained significant in multivariate analysis. Plausibly, organomegaly at diagnosis reflects the presence of a high mass of lymphoblasts in the organism, this can decrease the ratio of administered drugs to leukemic cell burden, favoring relapse. CCG also reported visceromegalies at diagnosis as a clinical predictor, but only for ICNS relapse [Citation36]. Thus, in our children organomegaly was as good a predictor as NCI Rome risk criteria, something that may be helpful in low-income countries where cytogenetics, MRD and additional sophisticated and expensive follow-up procedures are not always available. We did not find a statistical relation between a positive MRD and relapse, in contrast to a study that reported lower EFS when MRD was positive [Citation7]. When analyzing our high-risk patients, those with positive MRD after induction to remission therapy did not present an increased risk to relapse, in contrast to recent results reported by Children's Oncology Group in its ALL0232 study [Citation38]. A probable explanation is that most of our children belonged to the high-risk group, already making them strong candidates for relapse.
Relapse was higher, presented at an earlier time, occurred at an unusually high frequency in the CNS and survival rates were lower in our children compared to those in high-income countries; relapse thus remains the biggest threat for ALL children attaining CR after induction to remission therapy, and as such, must be addressed as the foremost contemporary challenge remaining to solve for pediatric hematologists worldwide. This effort should take advantage of recent advances in the molecular knowledge of recurrent relapse-associated pathways and the clinical or preclinical availability of drugs targeting these pathways, as recently reviewed [Citation10].
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
José Carlos Jaime-Pérez http://orcid.org/0000-0001-6804-9095
David Gómez-Almaguer http://orcid.org/0000-0002-0460-6427
References
- Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972
- Pérez-Saldivar ML, Fajardo-Gutiérrez A, Bernáldez-Ríos R, et al. Childhood acute leukemias are frequent in Mexico City: descriptive epidemiology. BMC Cancer. 2011;11:355. doi: 10.1186/1471-2407-11-355
- Abboud MR, Ghanem K, Muwakkit S. Acute lymphoblastic leukemia in low and middle-income countries: disease characteristics and treatment results. Curr Opin Oncol. 2014;26:650–655. doi: 10.1097/CCO.0000000000000125
- Vecchi V, Pession A, Paolucci G, et al. Risk-directed therapy for childhood acute lymphoblastic leukemia. results of the associazione italiana ematologia oncologia pediatrica ‘82 studies. Cancer. 1993;72:2517–2524. doi: 10.1002/1097-0142(19931015)72:8<2517::AID-CNCR2820720834>3.0.CO;2-1
- Ceppi F, Cazzaniga G, Colombini A, et al. Risk factors for relapse in childhood acute lymphoblastic leukemia: prediction and prevention. Expert Rev Hematol. 2015;8:57–70. doi: 10.1586/17474086.2015.978281
- Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a children’s oncology group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837
- Eckert C, von Stackelberg A, Seeger K, et al. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia – long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49:1346–1355. doi: 10.1016/j.ejca.2012.11.010
- Saarinen-Pihkala UM, Parto K, Riikonen P, et al. RALLE pilot: response-guided therapy for marrow relapse in acute lymphoblastic leukemia in children. J Pediatr Hematol Oncol. 2012;34:263–270. doi: 10.1097/MPH.0b013e3182352da9
- Irving JA. Towards an understanding of the biology and targeted treatment of paediatric relapsed acute lymphoblastic leukaemia. Br J Haematol. 2016;172:655–666. doi: 10.1111/bjh.13852
- Malempati S, Gaynon PS, Sather H, et al. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children’s Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800–5807. doi: 10.1200/JCO.2007.10.7508
- Locatelli F, Schrappe M, Bernardo ME, et al. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120:2807–2816. doi: 10.1182/blood-2012-02-265884
- Jimenez-Hernandez E, Jaimes-Reyes EZ, Arellano-Galindo J, et al. Survival of Mexican children with acute lymphoblastic leukaemia under treatment with the protocol from the Dana-Farber Cancer Institute 00-01. BioMed Res Int. 2015;2015: 576950.
- Mushtaq N, Fadoo Z, Naqvi A. Childhood acute lymphoblastic leukaemia: experience from a single tertiary care facility of Pakistan. J Pak Med Assoc. 2013;63:1399–1404.
- Campana D. Progress of minimal residual disease studies in childhood acute leukemia. Curr Hematol Malig Rep. 2010;5:169–176. doi: 10.1007/s11899-010-0056-8
- Sirvent N, Suciu S, Rialland X, et al. Prognostic significance of the initial cerebro-spinal fluid (CSF) involvement of children with acute lymphoblastic leukaemia (ALL) treated without cranial irradiation: results of European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group study 58881. Eur J Cancer. 2011;47:239–247. doi: 10.1016/j.ejca.2010.10.019
- Barredo JC, Devidas M, Lauer SJ, et al. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol. 2006;24:3142–3149. doi: 10.1200/JCO.2005.03.3373
- Lund B, Åsberg A, Heyman M, et al. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2011;56:551–559. doi: 10.1002/pbc.22719
- Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983
- Domenech C, Mercier M, Plouvier E, et al. First isolated extramedullary relapse in children with B-cell precursor acute lymphoblastic leukaemia: results of the Cooprall-97 study. Eur J Cancer. 2008;44:2461–2469. doi: 10.1016/j.ejca.2008.08.007
- Wood AJJ, Pui C-H, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. doi: 10.1056/NEJM199808273390907
- Jaime-Perez JC, López-Razo ON, García-Arellano G, et al. Results of treating childhood acute lymphoblastic leukemia in a low-middle income country: 10 year experience in northeast Mexico. Arch Med Res. 2016;47:668–676.
- Drachtman RA, Masterson M, Shenkerman A, et al. Long-term outcomes for children with acute lymphoblastic leukemia (ALL) treated on The Cancer Institute of New Jersey ALL trial (CINJALL). Leuk Lymphoma. 2016;57:2275–2280. doi: 10.3109/10428194.2016.1141406
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452
- Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc A Gen. 1972;135:185–207. doi: 10.2307/2344317
- Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220.
- Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a children’s oncology group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251
- Kamps WA, van der Pal-de Bruin KM, Veerman AJP, et al. Long-term results of Dutch childhood oncology group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–319. doi: 10.1038/leu.2009.258
- Makiya M. Tratamiento de la Leucemia Linfoblástica Aguda Pediátrica Recaída. Hematología. 2013;17:82–88.
- Campbell B, Salgado M, Quintana B, et al. Mejoría en el pronóstico de la leucemia linfoblástica aguda en niños de un país en desarrollo: Resultados del protocolo nacional chileno PINDA 87. Rev Chil Pediatr. 1999;70:405–414.
- Kuchler H, Buriot D, Maier M, et al. Acute lymphoblastic leukemia in childhood: importance of sex as a prognostic factor (author’s transl). Arch Fr Pediatr. 1982;39:17–21.
- Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep. 2015;4:4227. doi: 10.1038/srep04227
- Orgel E, Genkinger JM, Aggarwal D, et al. Association of body mass index and survival in pediatric leukemia: a meta-analysis. Am J Clin Nutr. 2016;103:808–817. doi: 10.3945/ajcn.115.124586
- Jaime-Pérez JC, González-Llano O, Herrera-Garza JL, et al. Assessment of nutritional status in children with acute lymphoblastic leukemia in Northern Mexico: a 5-year experience. Pediatr Blood Cancer. 2008;50:506–508. doi: 10.1002/pbc.21397
- Stary J, Zimmermann M, Campbell M, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. 2014;32:174–184. doi: 10.1200/JCO.2013.48.6522
- Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108:1165–1173. doi: 10.1182/blood-2005-12-011809
- Gaynon PS, Qu RP, Chappell RJ, et al. Survival after relapse in childhood acute lymphoblastic leukemia: impact of site and time to first relapse – the Children’s Cancer Group experience. Cancer. 1998;82:1387–1395. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1387::AID-CNCR24>3.0.CO;2-1
- Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126:964–971. doi: 10.1182/blood-2015-03-633685