ABSTRACT
Objectives: Increasing numbers of clinical studies have been carried out to investigate the therapeutic effect of Ofatumumab for patients with chronic lymphocytic leukemia (CLL) but no studies have yet reported a pooled estimate of the treatment effect. We performed a meta-analysis of evidence from 13 clinical trials to assess effectiveness and safety of Ofatumumab-based therapy in patients with CLL.
Methods: Relevant publications from PubMed, Web of Science, Embase, and ClinicalTrials.gov were searched. The primary efficacy outcomes were progression-free survival (PFS) and overall survival (OS). The second endpoint was the adverse events.
Results: The pooled efficacy analysis showed that there were no significant difference in PFS [hazard ratios (HR) = 0.88, 95% confidence interval (CI) = 0.47–1.63, p = 0.677, I2 = 94.9%] and OS (HR = 0.97, 95% CI = 0.70–1.36, p = 0.878, I2 = 58.7%) between Ofatumumab-based therapy and non-Ofatumumab therapy. The pooled toxicity analysis showed that Ofatumumab-based therapy was associated with an increased risk of infusion-related reaction but decreased risk of thrombocytopenia and anemia compared with non-Ofatumumab-based therapy. Moreover, infections, and infusion-related reaction occurred more frequently in participants with single Ofatumumab studies.
Discussion: Our analysis showed PFS was statistically significantly improved with Ofatumumab-based treatments (including Ofatumumab alone, Ofatumumab plus chemotherapy) for CLL compared with observation or chemotherapy-based regimen groups. Ofatumumab had no statistically significant improvement on the OS of patients with CLL. The Ofatumumab-based therapy could generally decrease the risk of adverse effects except infusion-related reaction and infections.
Introduction
Chronic lymphocytic leukemia (CLL) is a hematologic neoplasm and is characterized by progressive accumulation of mature B cells in the blood, lymph nodes, bone marrow, spleen, and liver [Citation1,Citation2]. CLL accounts for approximately 20–30% of all leukemias [Citation3]. CLL primarily affects people older than 50 years of age, and the median age at the time of diagnosis is 65–70 years [Citation4]. Patients with CLL present with a wide range of symptoms and signs including enlarged lymph nodes, liver, or spleen, fatigue, loss of appetite or early satiety, recurring infection, and abnormal bruising (late-stage symptom) [Citation5]. Patients with early-stage CLL are not treated with chemotherapy until symptoms start to appear or there is evidence of rapid progression of disease [Citation6]. CLL remains incurable with standard therapies which include mono- or polychemotherapies (i.e. fludarabine, cyclophosphamide, chlorambucil), usually plus monoclonal anti-CD20 antibodies (i.e. rituximab, Ofatumumab, obinutuzumab) [Citation7,Citation8]. Ofatumumab is a human IgG1κ monoclonal antibody that binds to a membrane-proximal epitope of CD20 molecule expressed on the surface of B lymphocytes. Ofatumumab, the second-generation anti-CD20 antibody, induces cell lysis through complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity. Ofatumumab is approved as monotherapy and in combination with chlorambucil for the treatment of fludarabine- and alemtuzumab-refractory CLL patients and previously untreated CLL patients, respectively. In particular, Ofatumumab received Food and Drug Administration (FDA) accelerated approval based on a surrogate endpoint, such as response rate or progression-free survival (PFS) [Citation9,Citation10]. Enforcement of postmarketing studies is therefore of critical importance to verify the drug’s clinical benefit (i.e. overall survival (OS)) and safety. Therefore, our meta-analysis aims to provide more comprehensive and up-to-date evidence on the efficacy and safety of Ofatumumab-based therapy for patients with CLL.
Methods
Literature and search strategy
PubMed, Web of Science, Embase, and ClinicalTrials.gov were searched for eligible articles and clinical trial information. The search strategy used combinations of the following key terms: ‘Ofatumumab’ and ‘chronic lymphocytic leukemia’ and ‘clinical study’ or ‘clinical trial’ (The literature search was updated on 30 November 2016). The reference lists of reviews and retrieved studies and included trials were hand-searched for potentially relevant citations.
Inclusion and exclusion criteria
Eligible studies had to meet the following criteria: (1) clinical trials evaluating the efficacy and safety of Ofatumumab-based therapy in patients with chronic lymphocytic leukemia whether they had control groups or not; (2) if multiple articles covered the same study population, the study with the most recent and complete data was used. The exclusion criteria were: (1) duplicate publications and (2) comments, case reports, abstract and review articles (including meta-analysis).
Data extraction
The following data were extracted: (1) first author’s name, (2) year of publication, (3) trial phase, (4) ClinicalTrials.gov Identifier, (5) interventions plan, (6) number of subjects, (7) hazard ratios (HRs) for PFS or OS, and (8) the numbers of selected adverse events. Adverse events evaluated in this analysis included neutropenia, cough, pneumonia, thrombocytopenia, anemia, infusion-related reaction, and infections. Data were extracted from all eligible publications and ClinicalTrials.gov independently by two authors and disagreement was resolved by discussion until consensus was reached.
Statistical analysis
For efficacy analysis, when available, HR and its 95% CI from different studies were pooled to calculate the PFS and OS benefit of Ofatumumab-based therapy and non-Ofatumumab therapy in chronic lymphocytic leukemia.
For toxicity analysis, the principal measures of major adverse events (both all grade and grade ≥ 3) for controlled trials were relative risk (RR) and corresponding 95% CI. Additionally, we calculated the proportion and derived 95% CI of adverse events for single-arm studies.
Cochran’s chi-square-based Q statistic test was performed to evaluate possible heterogeneity across studies. If heterogeneity existed (p < 0.1) among studies, the random effects model was used [Citation11]; otherwise, the fixed effects model was adopted [Citation12]. I2 statistic was calculated to quantify the proportion of the total heterogeneity among studies. Generally, I2 values of 25, 50, and 75% were used as evidence of low, moderate, and high heterogeneity, respectively.
Publication bias was assessed by funnel-plot analysis and Egger’s test [Citation13]. p < 0.05 was considered statistically significant. Data analyses were performed using STATA version 14.0 (Stata Corporation, College Station, TX, U.S.A.).
Results
Characteristics of the studies
A total of 460 records were obtained through the electronic database (219 in PubMed, 23 in Embase, 155 in Web of Science, and 63 in the ClinicalTrials.gov) in 30 November 2016. 65 unique articles were screened after 126 duplicates and 315 irrelevant reports were removed. Based on the inclusion criteria described above, 13 articles were finally included for meta-analysis [Citation14–26]. The detailed flow chart of the exclusion and inclusion process was presented in . The characteristics of included 13 trials were listed in . Eligible trials included five randomized controlled trials and eight single-arm studies. For single-arm trials, we calculated the incidence of adverse events.
Table 1. List of study characteristics.
Efficacy analysis
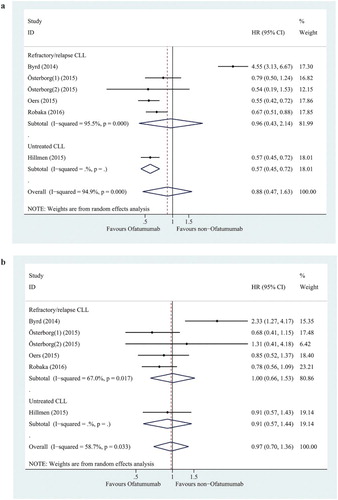
HR and 95% CI data of disease progression or survival were reported in six of the randomized controlled trials. To evaluate the differences of Ofatumumab on the efficacy of both upfront and relapsed CLL, studies have been classified into two subgroups; five studies with refractory/relapse CLL and one study with untreated CLL.
The overall analysis and refractory/relapse CLL subgroup analysis indicated no statistically significant improved PFS in the Ofatumumab group compared to the non-Ofatumumab group (HR = 0.88, 95% CI = 0.47–1.63, p = 0.677; HR = 0.96, 95% CI = 0.43–2.14, p = 0.915; respectively) ((a)). The PFS was significantly improved in the Ofatumumab group compared to the non-Ofatumumab group (HR = 0.57, 95% CI = 0.45–0.72, p < 0.0001) in untreated CLL subgroup analysis ((a)).
The overall analysis indicated no significant improvement in OS in the Ofatumumab group compared to the non-Ofatumumab group (HR = 0.97, 95% CI = 0.70–1.36, p = 0.878). Neither the refractory/relapse CLL subgroup nor the untreated CLL subgroup revealed a significant improvement in OS in the Ofatumumab group compared to the non-Ofatumumab group (HR = 1.00, 95% CI = 0.66–1.53, p = 0.567; HR = 0.91, 95% CI = 0.57–1.43, p = 0.666; respectively) ((b)).
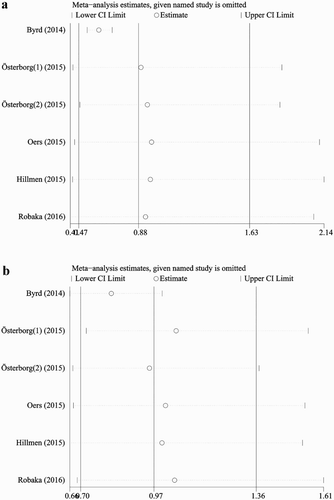
There was a significant and moderate level of heterogeneity between sample estimates for PFS and OS expressed as HR (PFS: I2, 94.9%; OS: I2, 58.7%), respectively. Sensitivity analysis was conducted to assess the influence of each study on the overall pooled HR ((a,b). The exclusion of Byrd et al. (2014) study made the biggest drop for heterogeneity values and significant difference in PFS between Ofatumumab-based therapy and non-Ofatumumab therapy was detected. However, still no significant difference was observed in OS between Ofatumumab-based therapy and non-Ofatumumab therapy ().
Table 4. Sensitivity analyses of HRs and 95% CI for PFS and OS in control-arm trials.
Safety analysis
All the included articles reported adverse events. After evaluating the all-grade and grade ≥ 3 adverse events, we found that the most common events included infections, neutropenia, infusion-related reaction, anemia, thrombocytopenia, cough, and pneumonia. Control trials were to determine the contribution of Ofatumumab in adverse events and single-arm studies were analyzed to calculate the adverse event rates. The results were presented in and .
Table 2. Comparative adverse events of Ofatumumab-based group versus non-Ofatumumab group.
Table 3. Adverse events rates of single-arm trials.
For control-arm studies, we analyzed infusion-related reaction, neutropenia, cough, infections, pneumonia, thrombocytopenia, and anemia with RR values. According to I2, random model was used in all-grade neutropenia, cough, infusion-related reaction, and infections, and grade ≥ 3 neutropenia. Among all-grade adverse events, infusion-related reaction (RR = 8.619, 95% CI: 1.818–40.859; p = 0.007), anemia (RR = 0.697, 95% CI: 0.547–0.887; p = 0.003), and thrombocytopenia (RR = 0.758, 95% CI: 0.621–0.925; p = 0.006) were statistically significant. Ofatumumab may play a dominant role in infusion-related reaction with the highest RR value. Similarly, grade ≥ 3 thrombocytopenia (RR = 0.570, 95% CI: 0.414–0.784; p = 0.001) and grade ≥ 3 infusion-related reaction (RR = 7.765, 95% CI: 3.423–17.105; p = 0.000) appeared statistically significant ().
With regard to single-arm trials, among all-grade adverse events, infections occurred more frequently in participants with the overall event rate being 0.482 (95% CI: 0.247–0.718; p = 0.000). The overall event rate of infusion-related reaction was 0.385 (95% CI: 0.088–0.683; p = 0.011). Neutropenia showed an overall event rate of 0.219 (95% CI: 0.126–0.312; p = 0.000). Anemia and thrombocytopenia occurred less frequently. Despite the high rates of adverse events, severe adverse events (grade ≥ 3) were relatively rare. Severe infections and neutropenia occurred in 0.242 (95% CI: 0.142–0.341; p = 0.000) and 0.120 (95% CI: 0.091–0.148; p = 0.000) of participants, respectively. Others seldom happened ().
Potential publication bias
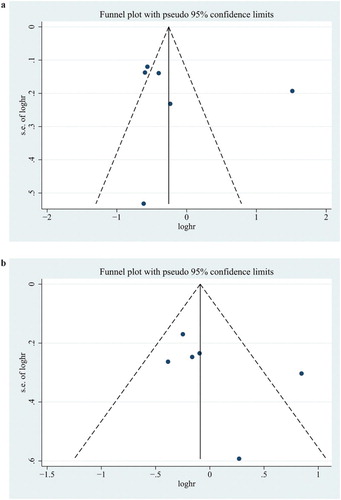
Funnel plots and Egger’s test were performed to access the publication bias of literatures. As showed in , the shape of the funnel plot did not reveal obvious asymmetry. Then, the Egger’s test was used to provide statistical evidence of funnel plot symmetry. No publication bias was observed in the statistical results (all p > 0.05).
Discussion
We systematically reviewed the international paper and combined the data from all published relevant clinical trials to evaluate the safety and efficacy of the novel anti-CD20 antibody Ofatumumab for relapsed/refractory chronic lymphocytic leukemia.
Meta-analysis of PFS and OS that included six trials demonstrated no advantage of Ofatumumab-based treatment compared with non-Ofatumumab regimens for CLL. Six trials reported PFS data. Five trials demonstrated a significant improved PFS with Ofatumumab-based treatment (including Ofatumumab alone, Ofatumumab plus chemotherapy) compared to non-Ofatumumab therapy (including observation or chemotherapy-based regimen). Six trials provided data on OS. Five trials compared Ofatumumab-based treatment group with observation or chemotherapy without Ofatumumab groups and found that there was no statistical difference on OS between the groups (all p > 0.05). Actually, Ofatumumab-based treatment was not shown in these five trials to significantly prolong OS.
A high and moderate level of statistical heterogeneity existed in the meta-analysis of PFS and OS, respectively. The sensitivity analysis results showed that Byrd et al. (2014) study was the source of heterogeneity [Citation14]. PFS was significantly improved for CLL patients randomized to ibrutinib (HR, 0.22; 95% CI, 0.15–0.32; p < 0.001) compared with the Ofatumumab group, as was OS (HR 0.43; 95% CI, 0.24–0.79; p = 0.005). However, the difference for PFS between Ofatumumab-based and non-Ofatumumab therapy was significant, but Ofatumumab still had no statistically significant OS benefit compared with placebo or observation after the Byrd et al. (2014) study was excluded [Citation14]. The results are consistent with an earlier report that evaluated the OS for many cancer drugs approved by the FDA [Citation27,Citation28].
Ofatumumab has shown a favorable toxicity profile. The most common adverse events include infusion-related reactions, infections and neutropenia. However, Ofatumumab-based therapy had lower risk of thrombocytopenia, anemia at all grades than any other therapy. The relative safety profiles of Ofatumumab in this meta-analysis are consistent with other earlier reports of review evaluating the safety profile of Ofatumumab for treating chronic lymphocytic leukemia [Citation29].
Conclusion
This systematic literature review with meta-analysis suggests that Ofatumumab-based treatments (including Ofatumumab alone, Ofatumumab plus chemotherapy) may be able to effectively improve the PFS, but does not have a statistically significant OS benefit for CLL patients in comparison with observation or chemotherapy-based regimen groups. The Ofatumumab-based therapy could generally decrease the risk of adverse effects except infusion-related reaction and infections. This study confirmed the efficacy and safety of Ofatumumab-based therapy in patients with CLL.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005 Feb 24;352(8):804–815. doi: 10.1056/NEJMra041720
- D'Arena G, Di Renzo N, Brugiatelli M, et al. Biological and clinical heterogeneity of B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2003 Feb;44(2):223–228. doi: 10.1080/1042819021000035756
- Redaelli A, Laskin BL, Stephens JM, et al. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl). 2004 Jul;13(3):279–287. doi: 10.1111/j.1365-2354.2004.00489.x
- Dores GM, Anderson WF, Curtis RE, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007 Dec;139(5):809–819. doi: 10.1111/j.1365-2141.2007.06856.x
- Elphee EE. Caring for patients with chronic lymphocytic leukemia. Clin J Oncol Nurs. 2008 Jun;12(3):417–423. doi: 10.1188/08.CJON.417-423
- Evans J, Ziebland S, Pettitt AR. Incurable, invisible and inconclusive: watchful waiting for chronic lymphocytic leukaemia and implications for doctor-patient communication. Eur J Cancer Care (Engl). 2012 Jan;21(1):67–77. doi: 10.1111/j.1365-2354.2011.01278.x
- Messori A, Fadda V, Maratea D, et al. First-line treatments for chronic lymphocytic leukaemia: interpreting efficacy data by network meta-analysis. Ann Hematol. 2015 Jun;94(6):1003–1009. doi: 10.1007/s00277-015-2310-6
- Bauer K, Rancea M, Roloff V, et al. Rituximab, ofatumumab and other monoclonal anti-CD20 antibodies for chronic lymphocytic leukaemia. Cochrane Database Syst Rev. 2012 Nov 14;11:CD008079.
- Lemery SJ, Zhang J, Rothmann MD, et al. U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res. 2010 Sep 01;16(17):4331–4338. doi: 10.1158/1078-0432.CCR-10-0570
- Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010 Apr 01;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015 Nov;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629
- Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014 Jul 17;371(3):213–223. doi: 10.1056/NEJMoa1400376
- Österborg A, Udvardy M, Zaritskey A, et al. Phase III, randomized study of ofatumumab versus physicians’ choice of therapy and standard versus extended-length ofatumumab in patients with bulky fludarabine-refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2016 Sep;57(9):2037–2046. doi: 10.3109/10428194.2015.1122783
- van Oers MH, Kuliczkowski K, Smolej L, et al. Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015 Oct;16(13):1370–1379. doi: 10.1016/S1470-2045(15)00143-6
- Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015 May 09;385(9980):1873–1883. doi: 10.1016/S0140-6736(15)60027-7
- Robak T, Warzocha K, Govind Babu K, et al. Ofatumumab plus fludarabine and cyclophosphamide in relapsed chronic lymphocytic leukemia: results from the COMPLEMENT 2 trial. Leuk Lymphoma. 2016 Oct;12:1–10.
- Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 25 Oct 2007;111(3):1094–1100. doi: 10.1182/blood-2007-09-111781
- Wierda WG, Padmanabhan S, Chan GW, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011 Nov 10;118(19):5126–5129. doi: 10.1182/blood-2011-04-348656
- Ogawa Y, Ogura M, Suzuki T, et al. A phase I/II study of ofatumumab (GSK1841157) in Japanese and Korean patients with relapsed or refractory B-cell chronic lymphocytic leukemia. Int J Hematol. 2013 Aug;98(2):164–170. doi: 10.1007/s12185-013-1393-x
- Ogura M, Hatake K, Tobinai K, et al. Phase I study of ofatumumab, a human anti-CD20 antibody, in Japanese patients with relapsed or refractory chronic lymphocytic leukemia and small lymphocytic lymphoma. Jpn J Clin Oncol. 2013 May;43(5):466–475. doi: 10.1093/jjco/hyt022
- Österborg A, Wierda WG, Mayer J, et al. Ofatumumab retreatment and maintenance in fludarabine-refractory chronic lymphocytic leukaemia patients. Br J Haematol. 2015 Jul;170(1):40–49. doi: 10.1111/bjh.13380
- Patton WN, Lindeman R, Butler AC, et al. An open-label, single-arm, phase 1 study to assess biomarker effects, efficacy and safety of ofatumumab in patients with refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(10):2819–2825. doi: 10.3109/10428194.2015.1014357
- Moreno C, Montillo M, Panayiotidis P, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a phase IV, non-interventional, observational study from the European research initiative on chronic lymphocytic leukemia. Haematologica. 2015 Apr;100(4):511–516. doi: 10.3324/haematol.2014.118158
- Flinn IW, Ruppert AS, Harwin W, et al. A phase II study of two dose levels of ofatumumab induction followed by maintenance therapy in symptomatic, previously untreated chronic lymphocytic leukemia. Am J Hematol. 2016 Oct;91(10):1020–1025. doi: 10.1002/ajh.24468
- Rupp T, Zuckerman D. Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med. 2017 Feb;177(2):276–277.
- Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: An analysis of 5 years of US food and drug administration approvals. JAMA Intern Med. 2015 Dec;175(12):1992–1994. doi: 10.1001/jamainternmed.2015.5868
- Korycka-Wołowiec A, Wołowiec D, Robak T. Ofatumumab for treating chronic lymphocytic leukemia: a safety profile. Expert Opin Drug Saf. 2015;14(12):1945–1959. doi: 10.1517/14740338.2015.1113253