ABSTRACT
Objectives: Monoclonal B-cell lymphocytosis (MBL) is a precursor state of chronic lymphocytic leukemia (CLL) with peripheral lymphocytosis below 5 × 109/l. The diagnostic criteria exclude the presence of lymphadenopathy, organomegaly, infections, autoimmune diseases or any sign of a lymphoproliferative disorder. This prospective study was designed in order to evaluate the frequency of MBL in blood donors in Turkey.
Methods: The diagnosis of MBL was identified by flow cytometry method based on the International Familial CLL Consortium Report. A total of 999 volunteers [median age 34 (18–78) years; male/female: 705/294] were included in the study.
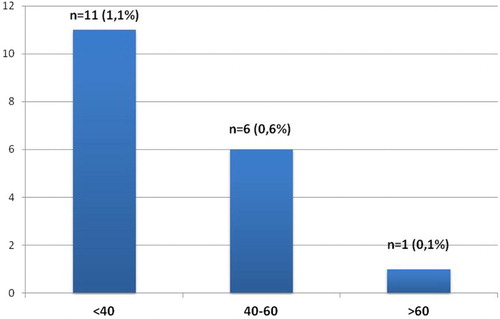
Results: Monoclonal B-cell lymphocytosis was demonstrated in 18 cases (1.8%). A total of 16 cases (1.6%) was evaluated as CLL-like MBL, while 2 (0.2%) had a non-CLL-like phenotype. The subjects were divided into three groups according to age, as <40 years, 40–60 years and >60 years. The prevalence of MBL was 1.1% below 40 years, 0.6% between 40 and 60 years and 0.1% in cases over 60 years, without statistical significance (p > 0.05).
Discussion: The sensitivity of the flow cytometry method is essential and may be responsible for the variations in the prevalence of MBL in different populations which can also be attributed to study design, higher detection rates in the elderly and families with genetic predisposition to CLL.
Conclusion: Large population-based studies and standardized laboratory methods are needed to determine the potential risk factors of progression to CLL, including molecular markers and genetic profile.
Introduction
Chronic lymphocytic leukemia (CLL) is a clonal lymphoproliferative disorder characterized by peripheral B-lymphocytosis (>5 × 109/l) co-expressing CD5, CD19, CD23, weak CD20, CD79b and surface immunoglobulin (sIg). Recently, a precursor state of CLL, monoclonal B-cell lymphocytosis (MBL), is defined as a reminiscent of the relationship between monoclonal gammopathy of undetermined significance (MGUS) and plasma cell myeloma. In a study by Landgren et al., 45 CLL patients were retrospectively analyzed and 44 patients had shown to have a prediagnostic B-cell clone 77 months before a CLL diagnosis. Monoclonal B-cell lymphocytosis indicates an asymptomatic presence of monoclonal B cells below 5 × 109/l [Citation1–11]. Based on the diagnostic criteria which were reported by the subcommittee of International Familial CLL Consortium, the primary distinguishing feature is the stable presence of a monoclonal B-cell population expressing a predominant CLL-like phenotype. Exclusion criteria include the presence of lymphadenopathy, organomegaly, infections, autoimmune diseases or any other features of a lymphoproliferative disorder [Citation5,Citation6,Citation10,Citation12–14]. The diagnostic criteria of MBL are updated in the World Health Organization (WHO) classification of lymphoid neoplasms in 2016. The previous criteria are generally confirmed, but low and high-count MBL definitions are particularly emphasized. Low-count MBL was defined as monoclonal B-cell count of <0.5 × 109/l and high-count as ≥0.5 × 109/l. Low-count MBL has a limited course, while high-count or clinical MBL (cMBL) has similar phenotypic, genetic and molecular features as Rai 0 CLL [Citation15–17].
Prevalence of MBL has been reported in a wide range, from 0.12 to 18.2%, in cytometric studies. This variability is considered to be mainly associated with study populations and sensitivity of laboratory methods. Using four-color flow cytometry, the prevalence of MBL was reported to be 3–5% in the general population. Monoclonal B-cell lymphocytosis is more common in the elderly and the prevalence increases with age reaching up to 5–9% in individuals over 60 years. However, with more sensitive methods, CLL-like cells were identified in more than 10% of adults aged 40 years or more [Citation18]. Monoclonal B-cell lymphocytosis is considered as a marker of inherited predisposition to CLL, as it was demonstrated in 13–18% of first-degree relatives of CLL patients in high risk families [Citation2–4,Citation7–11,Citation13,Citation14,Citation16,Citation19–28].
Based on the inadequate data regarding the prevalence of MBL in distinct ethnic groups and geographical areas, this prospective study was designed in order to evaluate the frequency of MBL in blood donors in Turkey.
Material and methods
A total of 999 volunteers [median age 34 (18–78) years; male/female: 705/294] were randomly selected among the blood donors who applied to Gazi University Blood Bank between 2010 and 2012. The diagnosis of MBL was identified based on the International Familial CLL Consortium Report which was recently confirmed by WHO criteria: (i) detection of a disease-specific immunophenotype or an overall kappa (κ)/lambda (λ) ratio >3:1 or κ/λ < 0.3:1, (ii) stable monoclonal B-cell population over a 3-month period (iii) absence of lymphadenopathy, organomegaly, autoimmune or infectious diseases and B lymphocyte counts <5 × 109/l [Citation11,Citation13,Citation17]. Nevertheless, in the current study, blood donors were tested for MBL only before donation and the stability of MBL in positive donors was not confirmed over a 3-month period.
Flow cytometry analysis by four-color staining was performed using peripheral blood collected into EDTA. All samples were analyzed on FACSCalibur flow cytometer (Becton Dickinson). Monoclonal antibodies IgM fluorescein isothiocyanate (FITC), IgD phycoerythrin (PE) and IgG FITC were purchased from BD Pharmingen and all the rest from Becton Dickinson. A total of 200,000 events per tube were acquired. Sequential gating strategy was used as previously described [Citation15,Citation25].
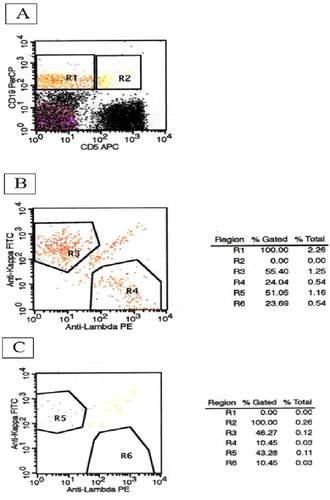
A two-step analysis method was used for MBL diagnosis. Initial panel consisted of CD5 allophycocyanin (APC) and CD19 peridin chlorophyll protein (PerCP)/anti κ FITC/anti λ PE. Cells were firstly evaluated by biparametric graphics based on CD5 and CD19 expressions ((A)). R1 and R2 represented CD5−CD19+ and CD5+CD19+ cells, respectively. Selected cells on R1 and R2 were then analyzed for κ and λ expressions. R3/R5 and R4/R6 showed κ and λ positive cells, respectively ((B,C)). Monoclonality was detected by light chain restriction which was defined as κ/λ > 3:1 or κ/λ < 0.3:1. Whole blood count and second panel were performed in cases with B-cell clonality in the initial panel.
Figure 1. Two-step analysis method for the detection of monoclonal B-cell lymphocytosis, initial panel (B: κ/λ: 2.3; C: κ/λ: 4.14).
Second panel was arranged based on the presence of lymphocytosis. If lymphocytosis was detected, the panel included CD3 FITC/CD3 control PE/CD19 PerCP; CD20 FITC/CD5 PE/CD19 PerCP; CD10 FITC/CD38 PE/CD19 PerCP; FMC7 FITC/CD22 PE/CD19 PerCP; CD11a FITC/CD23 PE/CD19 PerCP; IgM FITC/IgD PE/CD19 PerCP and IgG FITC/CD79b PE/CD19 PerCP. If lymphocyte count was normal, the analysis was switched to CD20 FITC/CD79b PE/CD19 PerCP; FMC7 FITC/CD23 PE/CD19 PerCP and CD5 APC profile.
Monoclonal B-cell lymphocytosis was classified as CLL-like (CD5+23+) and non-CLL (CD5 −) types.
Statistical analysis
Continuous variables were presented as median values, categorical variables as numbers and percentages. Categorical variables were compared using the Chi-squared test. Continuous variables in two groups were compared using Mann–Whitney U test. A threshold value of p < 0.05 was considered as statistically significant. The analysis was performed with SPSS 15.0 (SPSS Inc., Chicago, IL, U.S.A.).
The study was approved by the Ethical Committee of Gazi Medical School.
Results
Monoclonal B-cell lymphocytosis was demonstrated in 18 cases (1.8%). A total of 16 cases (1.6%) were evaluated as CLL-like MBL, while 2 (0.2%) had a non-CLL-like phenotype. Median leukocyte count was 7.5(3.16–21.22)×109/l. Median absolute lymphocyte count was found to be 2.2(0.88–6.5)×109/l, median lymphocyte percentage was 29.4% (9.6–63.5). Median absolute lymphocyte count in MBL cases was 2(1.2–2.7)×109/l and 2.2(0.88–6.5)×109/l in normal subjects (p > 0.05). Prevalence of MBL was not statistically different between male and female subjects (p > 0.05). Patient characteristics are summarized in .
Table 1. Patient characteristics.
The subjects were divided into three groups according to age as <40 years, 40–60 years and >60 years. The prevalence of MBL was 1.1% below 40 years, 0.6% between 40 and 60 years and 0.1% in cases over 60 years, without statistical significance (p > 0.05) ().
Discussion
In this prospective study, MBL was detected in 18 of 999 donors (1.8%) with a CLL-like phenotype predominance. Prevalence of MBL was lower in the elderly and similar between male and female subjects, without statistical significance.
Although MBL is reported to be more common among males in several studies [Citation27,Citation29], the prevalence was similar in male and female subjects in our study. Shim et al. investigated MBL in 2098 blood donors with six-color flow cytometry. Prevalence of MBL was found to be 7.1% in the study population. The prevalence was higher in men and increased with age [Citation27]. In a study of Mulligan et al., 414 MBL patients were investigated for demographics. The age distribution was found to be identical to CLL. However, male/female ratio of MBL was 1.09:1, in concordance with our results [Citation26].
In the current study, MBL was found to be more common in cases below 40 years, which may be associated with the relatively younger study population. As the median age of our study population was 34 years and there are only 21 cases over 60 years, current data may not be a feasible representative for the elderly population due to our small sample size.
The sensitivity of the flow cytometry method is essential for the detection of MBL in different populations. However, variations in prevalence can also be attributed to study design, higher detection rates in the elderly and families with genetic predisposition to CLL [Citation12,Citation30]. In a study from Spain, using eight-color flow cytometry approach, the overall prevalence of MBL was found to be 12% in 608 individuals who were over 40 years of age and the incidence was progressively increasing with age [Citation2,Citation30]. Another study from Italy reported a prevalence of 7.4% among 1725 individuals aged 18 years or older [Citation2,Citation31]. CLL-like phenotype was identified in 12 and 5.2% of patients, respectively. The overall prevalence of non-CLL-like subtype was 2.3% for both studies [Citation2,Citation30,Citation31]. In two studies using less sensitive flow cytometry methods, much lower prevalence rates of MBL, 0.14% among 5141 individuals aged 17 years or older and 0.6% among 1926 individuals aged 40 years or older, were reported. This lower prevalence was attributed to younger age and healthier profile of the study population which may also be an explanation for our similar results. Furthermore, as CLL is reported less common among Asian populations, a lower prevalence of MBL may be expected in these circumstances [Citation2,Citation12,Citation32].
Biological characteristics of CLL, such as mutational status of immunoglobulin heavy chain variable region (IGHV), cytogenetic abnormalities and molecular mutations were investigated in low-count MBL and cMBL. The frequency of IGHV-mutated cases is found to be significantly higher in individuals with low-count MBL. Low-count MBL cases represent genetic abnormalities associated with more favorable prognosis in CLL, such as deletion 13q. By contrast, the distribution of genetic abnormalities in cMBL is found to be comparable to CLL [Citation11].
Although most of the low-count cases are supposed to remain stable over time, individuals with cMBL are more likely to progress to CLL. Rossi et al. compared 123 cMBL and 154 Rai 0 CLL patients according to clinical and biological profile and outcome. They showed that cMBL has a more favorable clinical course when compared to Rai 0 CLL [Citation4,Citation10]. In a study by Rawstron et al., 1520 subjects were investigated for MBL which was detected in 5.1% of subjects with normal blood count and 13.9% with lymphocytosis. Absolute B-cell count was the only prognostic factor associated with progressive lymphocytosis. After a median follow-up of 6.7 years, a total of 28 cases (15%) with cMBL developed CLL. The annual risk of developing CLL requiring chemotherapy among subjects with cMBL was 1–2%, which is very similar to the rate of progression to myeloma in patients with MGUS [Citation1].
The transfer of MBL from donor to recipient may be observed in the course of allogeneic stem cell transplantation, however this may not be acceptable for blood transfusion in an immune-competent person [Citation15,Citation18,Citation33]. Further attempts are indicated to investigate the possible transference of MBL to blood transfusion recipients.
Large population-based studies and standardized laboratory methods are needed to determine the potential risk factors of progression to CLL including molecular markers and genetic profile. Long term follow-up is essential to observe the clinical course of MBL, especially cMBL patients should be monitored like early-stage CLL patients. Furthermore, understanding the early steps of the pathogenesis of CLL would also contribute to the identification of new therapeutic targets for this disease [Citation4,Citation6,Citation10,Citation21].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rawstron AC, Bennett FL, O’Connor SJM, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Eng J Med. 2008;359(6):575–583. doi: 10.1056/NEJMoa075290
- Shim YK, Middleton DC, Caporaso NE, et al. Prevalence of monoclonal B-cell lymphocytosis: a systematic review. Cytometry B Clin Cytom. 2010;78(Suppl 1):S10–S18. doi: 10.1002/cyto.b.20538
- Landgren O, Albitar M, Ma Wanlong, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Eng J Med. 2009;360:659–667. doi: 10.1056/NEJMoa0806122
- Rossi D, Sozzi E, Puma A, et al. The prognosis of clinical monoclonal B cell lymphocytosis differs from prognosis of Rai 0 chronic lymphocytic leukaemia and is recapitulated by biological risk factors. Br J Haematol. 2009;146:64–75. doi: 10.1111/j.1365-2141.2009.07711.x
- Rawstron AC. Occult B-cell lymphoproliferative disorders. Histopathology. 2011;58:81–89. doi: 10.1111/j.1365-2559.2010.03702.x
- Kern W, Bacher U, Haferlach C, et al. Monoclonal B-cell lymphocytosis is closely related to chronic lymphocytic leukaemia and may be better classified as early-stage CLL. Br J Haematol. 2012;157:86–96. doi: 10.1111/j.1365-2141.2011.09010.x
- Goldin LR, Slager SL, Caporaso NE. Familial chronic lymphocytic leukemia. Curr Opin Hematol. 2010;17(4):350–355. doi: 10.1097/MOH.0b013e328338cd99
- Slager SL, Kay NE. Familial CLL: what does it mean to me? Clin Lymphoma Myeloma. 2009;9(Suppl 3):S194–S197. doi: 10.3816/CLM.2009.s.011
- Goldin LR, Lanasa MC, Slager SL, et al. Common occurrence of monoclonal B-cell lymphocytosis among members of high risk CLL families. Br J Haematol. 2010;151(2):152–158. doi: 10.1111/j.1365-2141.2010.08339.x
- Mowery YM, Lanasa MC. Clinical aspects of monoclonal B-cell lymphocytosis. Cancer Control. 2012;19(1):8–17.
- Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early stage chronic lymphocytic leukemia: diagnosis, natural history and risk stratification. Blood. 2015;126(4):454–462. doi: 10.1182/blood-2015-02-585059
- Rachel JM, Zucker ML, Fox CM, et al. Monoclonal B cell lymphocytosis in blood donors. Br J Haematol. 2007;139:832–836. doi: 10.1111/j.1365-2141.2007.06870.x
- Marti GE, Rawstron AC, Ghia P, et al. Diagnostic criteria for monoclonal B lymphocytosis. Br J Haematol. 2005;130:325–332. doi: 10.1111/j.1365-2141.2005.05550.x
- Matos DM, Ismael SJ, Scrideli CA, et al. Monoclonal B-cell lymphocytosis in first degree relatives of patients with sporadic (non-familial) chronic lymphocytic leukaemia. Br J Haematol. 2009;147:339–346. doi: 10.1111/j.1365-2141.2009.07861.x
- Stetler-Stevenson M. Monoclonal B-cell lymphocytosis in donors. Blood. 2014;123(9):1281–1282. doi: 10.1182/blood-2014-01-546739
- Kalpadakis C, Pangalis GA, Sachanas S, et al. New insights into monoclonal B-cell lymphocytosis. BioMed Res Int. 2014;2014:1–11. doi: 10.1155/2014/258917
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of world health organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569
- Rawstron AC. Monoclonal B cell lymphocytosis – what does it really mean? Curr Hematol Malig Rep. 2013;8:52–59. doi: 10.1007/s11899-012-0144-z
- Rawstron AC, Yuille MR, Fuller J, et al. Inherited predisposition to CLL is detectable as subclinical monoclonal B-lymphocyte expansion. Blood. 2002;100:2289–2291. doi: 10.1182/blood-2002-03-0892
- De Tute R, Yuille M, Catovsky D, et al. Monoclonal B-cell lymphocytosis (MBL) in CLL families: substantial increase in relative risk for young adults. Leukemia. 2006;20:728–729. doi: 10.1038/sj.leu.2404116
- Goldin LR, Landgren O, Marti GE, et al. Familial aspects of chronic lymphocytic leukemia, monoclonal B-cell lymphocytosis (MBL) and related lymphomas. Eur J Clin Med Oncol. 2010;2(1):119–126.
- Marti G, Abbasi F, Raveche E, et al. Overview of monoclonal B-cell lymphocytosis. Br J Haematol. 2007;139:701–708. doi: 10.1111/j.1365-2141.2007.06865.x
- Brown JR. Inherited predisposition to chronic lymphocytic leukemia. Expert Rev Hematol. 2008;1(1):51–61. doi: 10.1586/17474086.1.1.51
- Marti GE, Carter P, Abbasi F, et al. B-cell monoclonal lymphocytosis and B-cell abnormalities in the setting of familial B-cell chronic lymphocytic leukemia. Cytometry B (Clin Cytom). 2003;52B:1–12. doi: 10.1002/cyto.b.10013
- Abbasi F, Longo NS, Lipsky PE, et al. B-cell repertoire and clonal analysis in unaffected first degree relatives in familial chronic lymphocytic leukaemia kindred. Br J Haematol. 2007;139:820–823. doi: 10.1111/j.1365-2141.2007.06857.x
- Mulligan CS, Thomas ME, Mulligan SP. Monoclonal B-lymphocytosis: demographics, nature and subclassification in 414 community patients. Leuk Lymphoma. 2011;52(12):2293–2298. doi: 10.3109/10428194.2011.598250
- Shim YK, Rachel JM, Ghia P, et al. Monoclonal B-cell lymphocytosis in healthy blood donors: an unexpectedly common finding. Blood. 2014;123(9):1319–1326. doi: 10.1182/blood-2013-08-523704
- Demirci T, Yegin ZA, Kurşunoğlu N, et al. Prevalence of monoclonal B lymphocytosis in first degree relatives of chronic lymphocytic leukemia patients in Turkey. Turk J Hematol. 2015;32(1):29–34. doi: 10.4274/tjh.2013.0288
- Ghia P, Caligaris-Cappio F. Monoclonal B-cell lymphocytosis: right track or red herring? Blood. 2012;119(19):4358–4362. doi: 10.1182/blood-2012-01-404681
- Nieto WG, Almeida J, Romero A, et al. Increased frequency (%12) of circulating CLL-like B-cell clones in healthy individuals using a high-sensitive multicolor flow cytometry approach. Blood. 2009;114:33–37. doi: 10.1182/blood-2009-01-197368
- Dagklis A, Fazi C, Sala C, et al. The immunoglobulin gene repertoire of low-count CLL-like MBL is different from CLL: diagnostic implications for clinical monitoring. Blood. 2009;114:26–32. doi: 10.1182/blood-2008-09-176933
- Shim YK, Vogt RF, Middleton D, et al. Prevalence and natural history of monoclonal and polyclonal B-cell lymphocytosis in a residential adult population. Cytometry B Clin Cytom. 2007;72B:344–353. doi: 10.1002/cyto.b.20174
- Hjalgrim H, Rostgaard K, Vasan SK, et al. No evidence of transmission of chronic lymphocytic leukemia through blood transfusion. Blood. 2015;126(17):2059–2061. doi: 10.1182/blood-2015-03-632844