ABSTRACT
Objective: To define the positron emission tomography/computed tomography (PET/CT) features of monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), a rare malignancy in European and North American populations and the most common form of primary intestinal T-cell lymphoma in Asian populations.
Methods: 18F-fluorodeoxyglucose (FDG) PET/CT findings of a cohort of MEITL patients were retrospectively analyzed.
Results: Eight men and four women with MEITL investigated by PET/CT at diagnosis and relapse were retrospectively analyzed. On presentation, the primary involved sites were the small bowel (N = 8), large bowel (N = 2), stomach (N = 1) and small and large bowels (N = 1). The uninvolved small bowel did not show increased FDG-avidity to suggest enteropathy. On presentation, lymph nodes and other organs were involved in seven cases (58%). The primary lesions were hypermetabolic except in one case, where the colonic lesion was eumetabolic. At relapse, the stomach and large bowel might be involved even if the primary tumours arose from the small bowel, and multiple extra-intestinal metastases occurred. Interestingly, thoracic structures and the brain were frequently involved (50% and 25% respectively).
Conclusion: These findings showed that in contrast to classical enteropathy-associated T-cell lymphoma, where the small bowel is the exclusive primary site (owing to its origin from coeliac disease) and distant metastases even during relapse are exceptional, MEITL might on presentation and during relapse involve any part of the gut, and metastasize to multiple extra-intestinal sites.
Introduction
Primary T-cell lymphoma of the gastrointestinal tract is a very rare and aggressive intestinal tumour of intraepithelial T-lymphocytes, accounting for <1% of all lymphomas [Citation1]. There are two different types, enteropathy-associated T-cell lymphoma (EATL, previously referred to as classical EATL) and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL, previously referred to as EATL type II) [Citation2]. EATL evolves from refractory coeliac disease. Therefore, pathological features of enteropathy including villous atrophy and crypt hyperplasia are found in the lymphoma [Citation1]. It constitutes 80% of intestinal T-cell lymphomas in European and North American populations, particularly in patients of North European descent. However, because coeliac disease does not exist in Asian people, EATL is unknown in this population.
Monomorphic epitheliotropic intestinal T-cell lymphoma is the other type of primary intestinal T-cell lymphoma [Citation2]. It accounts for the remaining 20% of cases in European and North American populations, particularly in Hispanic patients [Citation1,Citation2]. It is the exclusive form in Asia [Citation3–6]. Pathologically, lymphoma cells are medium-sized and monomorphic, expressing characteristically CD8, CD56 and MAT kinase [Citation6]. A significant proportion expresses the gamma delta T-cell receptor [Citation3,Citation4]. This lymphoma is not aetiologically or pathologically related to enteropathy [Citation7]. Therefore, in the revised World Health Organization classification of lymphoid malignancies [Citation2], MEITL has been designated as a separate clinicopathologic entity distinct from EATL.
Positron emission tomography/computed tomography (PET/CT) is currently considered the standard imaging modality for the diagnosis, staging and response assessment in Hodgkin lymphoma and other aggressive B-cell lymphomas [Citation8]. However, the role of PET/CT in less common lymphomas, particularly T-cell lymphomas, remains to be defined.
Data of PET/CT in intestinal T-cell lymphomas are very scanty, and the few cases previously reported were all EATL [Citation9–11]. Because MEITL is now a distinct entity, there is a clear need to define its imaging features. In this study, we report the PET/CT findings of a cohort of MEITL patients, so that more can be known for this new and rare disease entity.
Patients and methods
Patients
Consecutive patients with MEITL (their previous diagnostic label would have been type II EATL) treated between January 2008 and July 2016 were studied. The diagnosis was based on typical histopathological and immunophenotypical features [Citation1,Citation2]. Standard clinical and biochemical evaluations for staging and response assessment were undertaken. Treatment protocols evolved over this period and were heterogeneous.
Positron emission tomography/computed tomography protocol and analysis
Details of PET/CT protocol and analysis have been described previously [Citation12,Citation13]. Briefly, whole-body 18F-fluorodeoxyglucose (FDG) PET/CT from skull base to the upper third of the thighs was performed with a dedicated combined PET/CT scanner. Activity of FDG was 4.8 MBq kg−1, with an uptake time of 60 min. PET images were analyzed visually and quantitatively. Positive uptake was defined as increased metabolic activity in locations incompatible with normal anatomy or anatomical variant. Responses were assessed according to the 5-point Deauville score (DS). DS of ≤3 was considered to represent metabolic response. Standardized uptake value (SUV) was measured by normalization to the injected dose and lean body mass. Areas with maximum SUV (SUVmax) were identified as index lesions. For post-treatment evaluations, change in SUVmax (ΔSUVmax) was defined as (SUVmax at pre-treatment – SUVmax at post-treatment)/SUVmax at pre-treatment; regardless of whether the lesion with SUVmax at post-treatment corresponded to the pre-treatment index lesion.
Results
Patients
Thirteen patients with MEITL were treated. One patient did not have PET/CT scan performed. Twelve patients (8 men, 4 women) at a median age of 56 (39–70) years were analyzed (). Staging systems developed specifically for gastrointestinal lymphomas classified more patients as having advanced tumour (Lugano, stage II2–IV: 50%; Manchester, stage IIb–IV: 58%); which probably better reflected the actual disease extent than the Ann Arbor staging system (stage III–IV: 33%).
Table 1. Demographic and clinical features of 12 cases of MEITL.
Positron emission tomography/computed tomography features of primary presentation in the small bowel
The small bowel was primarily involved in four cases (ileum, N = 3; duodenum, N = 1; ). All evaluable lesions were FDG-avid, with SUVmax varying from 4.0 to 8.7. None of the cases showed FDG-avidity in the uninvolved small bowel to suggest co-existing enteropathy (). All cases were disseminated on presentation. Two cases (patients 4 and 11) had involvement of regional lymph nodes. One case (patient 8) spread to involve the biliary tract, and one case (patient 3) had multiple metastases involving the omentum, supraclavicular lymph nodes and the lung.
Figure 1. Patient 4, showing primary small bowel involvement. (A) Markedly hypermetabolic disease involving segments of the jejunum and ileum (arrows) without evidence of FDG-avidity in the remaining small bowel to suggest co-existing enteropathy. There were also hypermetabolic nodal disease (open arrow). (B) Axial image showing markedly hypermetabolic thickened and dilated jejunal loop in the upper abdomen. (C) Coronal image showing hypermetabolic diseased jejunal and ileal loops (arrows).
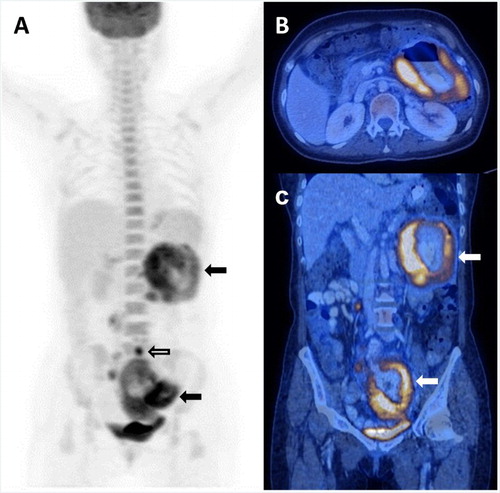
Table 2. PET/CT features of 12 patients with MEITL on diagnosis and at relapse.
Positron emission tomography/computed tomography features of primary presentation in the large bowel
The large bowel was primarily involved in two cases (patients 6 and 12). Interestingly, the lesion was eumetabolic in one case (patient 6) ((A)), without any evidence of dissemination. In the other case, regional lymph nodes were involved.
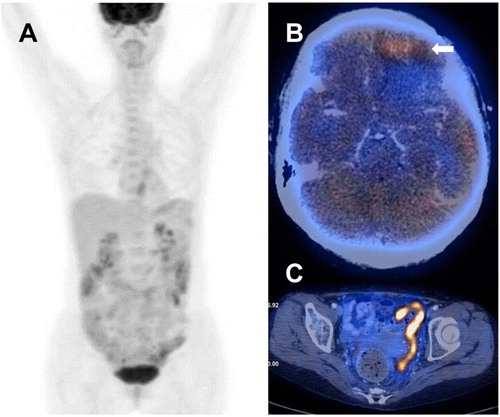
Figure 2. Patient 6, showing a change of eumetabolic lesions on presentation to hypermetabolic lesions during disease progression. (A) PET/CT at diagnosis, showing absence of hypermetabolic lesion, despite pathologically proven involvement of the colon. (B) Axial image at disease progression, showing hypermetabolic tumour at left frontal lobe (arrow). (C) Axial images at relapse, showing hypermetabolic sigmoid thickening with extension of disease to pelvic peritoneum.
Positron emission tomography/computed tomography features of primary presentation in the stomach
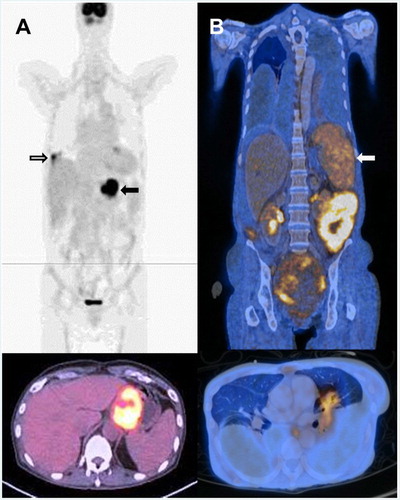
The stomach was primarily involved in one case (patient 10) ((A)), with SUVmax substantially higher at 12.1–14.4. The lung was involved ((A)), with a comparably high SUVmax of 8.5, whereas the oesophageal involvement had a lower SUVmax of 2.4 ((A)).
Figure 3. Involvement of extra-intestinal sites on presentation and relapse. (A) Patient 10 on presentation. Upper panel: hypermetabolic primary lesions in distal oesophagus and stomach (arrow). There was another metabolically active lesion in the lower lobe of right lung (open arrow). Lower panel: axial image showing hypermetabolic lesion in distal oesophagus and proximal stomach. (B) Patient 7 with primary lesion involving small bowel, and relapse involving other anatomical sites but not the small bowel. Upper panel: coronal image, showing involvement of the spleen (arrow). Lower panel: axial image showing hypermetabolic lesion in the left lung.
Positron emission tomography/computed tomography features of primary presentation in the small and large bowels
One case (patient 5) had concomitant involvement of the small and large bowels (both sites biopsy-proven). There was widespread involvement of multiple lymph nodes, pleura and lung.
Radiologic features in other cases on presentation
Two cases (patients 1 and 2) only had CT scans for staging. In patient 1, the disease was localized to the gastroduodenal junction. In patient 2, the lesion presented as an 8-cm circumscribed wall-off perforation in the abdomen communicating with the small bowel.
Treatment
Four patients received a methotrexate-containing regimen (methotrexate, ifosfamide, L-asparaginase, etoposide, dexamethasone) [Citation4], whereas six patients received a cisplatin-containing regimen (cisplatin, ifosfamide, gemcitabine, L-asparaginase, etoposide, dexamethasone). Two patients received CHOP (cyclophosphamide, adriamycin, vincristine, prednisolone).
Interim positron emission tomography/computed tomography
Nine patients had interim scan (after 2–3 cycles of treatment) (). Seven patients had metabolic complete response (mCR, DS: 1, N = 6; DS: 3, N = 1), and two patients had disease persistence (DS: ≥4). For the seven patients with interim mCR, two patients (cases 7 and 10) relapsed shortly afterwards and later succumbed to their illness; two patients (cases 4 and 6) had disease progression/relapse at end-of-treatment scan; one patient (case 8) died of complications during subsequent treatment; and only two patients (cases 11 and 12) maintained mCR at end-of-treatment scan. For the two patients showing disease persistence at interim, one patient achieved mCR at end-of-treatment scan (case 9), whereas the other patient (case 5) showed no improvement.
Table 3. PET/CT findings and outcome of 10 patients with MEITL.
Positron emission tomography/computed tomography features at progressive disease or relapse
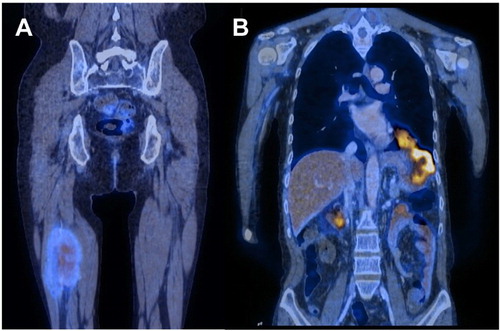
Five patients had PET/CT at PD or relapse. In four cases with primary small bowel tumour (), numerous sites were involved in three cases (patients 1, 2 and 7) including the thorax (N = 3), breast (N = 1), central nervous system (CNS) (N = 1), distant lymph nodes (N = 1), thigh (N = 1) ((A)) and liver and spleen (N = 1) ((B)). Interestingly, in one case with primary ileal involvement (patient 1), the lesion spread to the stomach at relapse ((B)). In one case (patient 4), the relapse was restricted to the small bowel. In the remaining case (patient 6) with colon as the primary site, the CNS was remarkably the only site that was involved in disease progression ((B)). It remained the only extra-intestinal site involved at relapse. Furthermore, although the primary colonic tumour was eumetabolic, metastatic lesions were hypermetabolic (appendix, SUVmax = 3.9; sigmoid colon: SUVmax = 12.4; basal ganglia: SUVmax = 4.4; left frontal lobe: SUVmax = 4.9) ((C)) ().
Figure 4. Patient 1, who had small bowel involvement on presentation but involvement of extra-intestinal sites on relapse. (A) Coronal image showing hypermetabolic lesion in the thigh. (B) Coronal image showing hypermetabolic lesions in stomach infiltrating through the left hemi-diaphragm to involve the left thoracic cavity.
Radiologic features in other cases at relapse
Patient 10 had disease relapse in the CNS that progressed rapidly. He did not have a PET/CT done at relapse.
Outcome and prognostic indicators
The median survival of the cohort was 13 (1–136) months. Seven patients completed the intended therapy, with six of them achieving mCR (). Patients with mCR on completion of treatment had a median survival of 14 (13–136) months, which was apparently better than the patient who could not achieve mCR (patient 4, survival 11 months), although a formal statistical evaluation was not possible owing to the small number of cases.
Discussion
This study is to date the largest reported series of imaging features in MEITL patients. Our findings clearly distinguished this lymphoma from classical EATL, and gave important information on the clinical and biological characteristics of this new disease entity.
Classical EATL always arises from the small bowel, and involvement of the rest of the gastrointestinal tract has not been convincingly shown [Citation9–11]. In MEITL, however, we showed with PET/CT that involvement of the oesophagus, stomach and large bowel occurred in 50% of cases (), both on presentation and at relapse. On PET/CT, classical EATL occurs as hypermetabolic lesions with high SUVmax, against a background of diffuse FDG uptake of lower SUVmax deriving from the underlying coeliac disease [Citation9,Citation10]. In one series of four cases, classical EATL arising from coeliac disease had SUVmax of 6.4–8.0, which was significantly higher than the background SUVmax of 2.2–4.6 of the inflammed gut [Citation9]. In our cases of MEITL, lesions appeared as discrete hypermetabolic lesions with no background activity, as enteropathy was not present. Although interpretation of the SUVmax of lesions depends on the values of the mediastinal blood pool or liver, the SUVmax values in MEITL were generally lower than those of aggressive B-cell lymphomas [Citation13].
When classical EATL relapses, extra-intestinal sites are rarely if ever involved. In a total of 15 cases of classical EATL examined with PET/CT [Citation9–11], only two cases had extra-intestinal involvement on relapse, presenting as mediastinal lymphadenopathy. Conversely, diverse extra-intestinal sites were involved during relapse in our cases.
Our results also provided novel observations in MEITL. It was not a gut-restricted lymphoma. On presentation and at relapse, many different anatomical sites were involved. Infiltration of thoracic structures (lung, pleura and diaphragm) occurred in 50% of our cases, which was surprisingly high when compared with that of merely 7% of thoracic involvement in aggressive diffuse large B-cell lymphoma (DLBCL) at any stage of illness [Citation14]. During disease progression and relapse, the CNS was another site commonly involved. At a frequency of 25%, CNS involvement in our cases was again more common than that of approximately 7% in DLBCL [Citation15]. The widespread involvement of extra-intestinal but non-nodal sites is reminiscent of the pattern observed in other aggressive extranodal T-cell lymphomas.
In conclusion, PET/CT scan findings have shown that MEITL is distinctly different from classical EATL in many aspects (). These observations are consistent with recent genetic studies that showed different mutational profiles in MEITL and classical EATL [Citation15]. Finally, the high frequency of extra-intestinal involvement in MEITL, particularly the CNS, suggests that high-dose chemotherapy may be needed to improve treatment outcome.
Table 4. Differences in PET/CT features between MEITL and classical EATL.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Elaine Lee http://orcid.org/0000-0002-0627-5297
References
- Isaacson PG, Chott A, Ott G, et al. Enteropathy-associated T-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of the haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008. p. 89–291.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569
- Chan JK, Chan AC, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent γδ T-cell receptor expression. Am J Surg Pathol. 2011;35(10):1557–1569. doi: 10.1097/PAS.0b013e318222dfcd
- Tse E, Gill H, Loong F, et al. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia lymphoma study group. Am J Hematol. 2012;87(7):663–668. doi: 10.1002/ajh.23213
- Tan SY, Chuang SS, Tang T, et al. Type II EATL (epitheliotropic intestinal T-cell lymphoma): a neoplasm of intra-epithelial T-cells with predominant CD8αα phenotype. Leukemia. 2013;27(8):1688–1696. doi: 10.1038/leu.2013.41
- Tan SY, Ooi AS, Ang MK, et al. Nuclear expression of MATK is a novel marker of type II enteropathy-associated T-cell lymphoma. Leukemia. 2011;25(3):555–557. doi: 10.1038/leu.2010.295
- Swerdlow SH, Jaffe ES, Brousset P, et al. International lymphoma study group. Cytotoxic T-cell and NK-cell lymphomas: current questions and controversies. Am J Surg Pathol. 2014;38(10):e60–e71. doi: 10.1097/PAS.0000000000000295
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32(27):3048–3058. doi: 10.1200/JCO.2013.53.5229
- Hoffmann M, Vogelsang H, Kletter K, et al. 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) for assessment of enteropathy-type T cell lymphoma. Gut. 2003;52(3):347–351. doi: 10.1136/gut.52.3.347
- Hadithi M, Mallant M, Oudejans J, et al. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. J Nucl Med. 2006;47(10):1622–1627.
- Casulo C, Schöder H, Feeney J, et al. 18F-fluorodeoxyglucose positron emission tomography in the staging and prognosis of T cell lymphoma. Leuk Lymphoma. 2013;54(10):2163–2167. doi: 10.3109/10428194.2013.767901
- Khong PL, Huang B, Lee EY, et al. Midtreatment 18F-FDG PET/CT scan for early response assessment of smile therapy in natural killer/T-cell lymphoma: a prospective study from a single center. J Nucl Med. 2014;55(6):911–916. doi: 10.2967/jnumed.113.131946
- Chan WK, Au WY, Wong CY, et al. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med. 2010;35(8):571–575. doi: 10.1097/RLU.0b013e3181e4dcbf
- Mian M, Wasle I, Gritsch S, et al. B cell lymphoma with lung involvement: what is it about? Acta Haematol. 2015;133(2):221–225. doi: 10.1159/000365778
- Villa D, Connors JM, Shenkier TN, et al. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046–1052. doi: 10.1093/annonc/mdp432
