ABSTRACT
Objectives: Although decreased thyroid function is negatively correlated with clinical outcomes in critically ill patients, its role in allogeneic haematopoietic cell transplantation (allo-HCT) has not been sufficiently described.
Methods: The associations between pre-conditioning thyroid hormone concentrations and transplant-related complications in 474 adult patients with haematologic malignancies who underwent myeloablative allo-HCT were assessed.
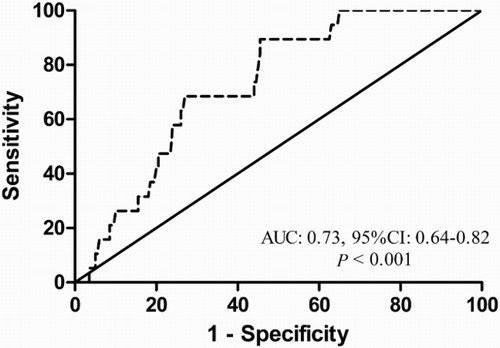
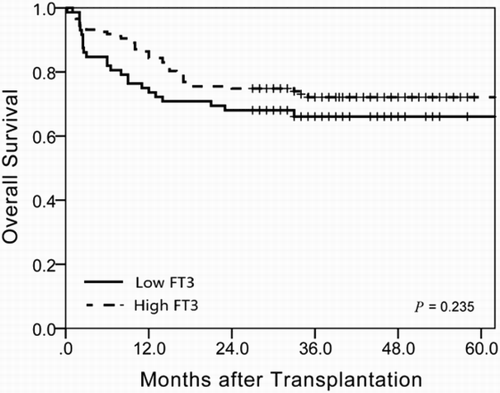
Results: A receiver-operating characteristic curve showed that the baseline serum-free triiodothyronine 3 (FT3) level had an excellent predictive value for non-relapse mortality (NRM) within 100 days in sibling HCT with an area under the curve of 0.73 [95% confidence interval (CI), 0.64–0.82]. With a cut-off value of 4.7 pmol/l, the sensitivity and specificity for early NRM were 68% and 73%, respectively. The cumulative incidences of early NRM within 100 days after sibling HCT were 14% (95% CI, 10–18%) in the low FT3 group and 6% (95% CI, 4–8%) in the high-FT3 group (p = 0.033). In multivariate analysis, a lower FT3 level was significantly associated with high early NRM (HR = 3.19, 95% CI, 1.13–9.03, p = 0.029). The difference was also significant at 3 years after HCT (24% vs. 14%, p = 0.046). Recipients with lower FT3 levels also had a trend towards a lower OS at 3 years after HCT (66% vs. 72%, p = 0.235), although the difference did not reach statistical significance.
Conclusion: A low FT3 level before conditioning may be a useful predictive biomarker for higher early NRM among patients undergoing myeloablative sibling transplantation.
Introduction
Allogeneic haematopoietic cell transplantation (allo-HCT) is a potentially life-saving treatment for many malignant haematologic disorders, but its application is hampered by considerable morbidity and mortality [Citation1]. Even after allo-HCT with nonmyeloablative or reduced-intensity conditioning (RIC) regimens, the non-relapse mortality (NRM) may range from 10% to 40% [Citation2,Citation3]. To determine the HCT risks, HCT comorbidity index (HCT-CI) which included 17 pretransplant comorbidities was developed to provide important assessment information [Citation4,Citation5]. However, the majority of patients undergoing allo-HCT had a low HCT-CI score. In addition to the intricate index, some simple pretransplant factors may significantly affect the outcome of patients after HCT [Citation6–8]. Therefore, there is a need to identify less complex but also reproducible predictors to better predict HCT complications.
Changes in the thyroid hormone levels, especially reduced serum-free triiodothyronine 3 (FT3) levels in the absence of an intrinsic thyroid disease, are highly prevalent in critical illness and chronic conditions [Citation9–11]. The altered thyroid hormone metabolism not only reflects the severity of the underlying disease, but it is also inversely correlated with endothelial activation and circulating markers of inflammation such as CRP and IL-6 [Citation12–14]. There is clinical evidence suggesting that a low FT3 level is a strong independent predictor of unfavourable clinical outcomes in many hospitalized patients, including patients with sepsis or septic shock, patients undergoing coronary artery bypass grafting and patients receiving kidney transplant with end-stage renal disease [Citation15–17]. To date, however, the prognostic importance of thyroid function for patients before allogeneic HCT has not been sufficiently described. In this study, we tested the hypothesis that reduced levels of pre-conditioning serum FT3 values would be predictive of increased NRM.
Patients and methods
Patients
This is a retrospective study of 474 adult patients with haematologic malignancies who underwent myeloablative allogeneic HCT in our haematology department between July 2011 and June 2014. High-risk diseases included acute leukaemia and lymphoma that was not in the first complete remission and chronic myeloid leukaemia that was not in the first chronic phase and myelodysplastic syndrome in refractory anaemia with excess blasts-II. All other patients were considered a standard risk. The study was approved by our hospital’s Ethics Committee.
Transplantation procedures
Details of the transplantation procedures have been published previously [Citation18]. Briefly, myeloablative conditioning regimens consisted of cyclophosphamide and either busulfan or total body irradiation. Rabbit antithymocyte globulin (Genzyme) was given at a dose of 10 mg/kg as an additional immunosuppressive measure in patients with transplantation from non-HLA-identical sibling donors. The stem cell source was either unmanipulated granulocyte colony-stimulating factor-mobilized bone marrow or peripheral blood stem cells from a matched sibling, unrelated donor or haplo-identical relative. Graft-versus-host disease (GVHD) prophylaxis consisted of the continuous infusion of cyclosporine A combined with short-term methotrexate. Recipients of non-HLA-identical sibling transplants also received mycophenolate mofetil. Pre-emptive therapy with ganciclovir was administered for cytomegalovirus prophylaxis for 10 days before transplantation, and standard prophylactic antibiotics were administered in accordance with the institutional guidelines.
Thyroid function assessment
The serum concentrations of FT3 (normal range, 3.1–6.8 pmol/l), free triiodothyronine 4 (FT4) (normal range, 12.0–22.0 pmol/l) and thyrotropin (TSH) (normal range, 0.3–4.2 mIU/l) were measured within 1 month before the start of the conditioning regimen with an electrochemiluminescence immunoassay with commercially available kits (Roche Diagnostics Shanghai Ltd, China) by a Cobas e 601 immunoassay analyzer (Roche Diagnostics Ltd, Switzerland). All intra- and inter-assay coefficients of variation were <10%.
Definition of transplant-related complications
Infection included microbiologically documented and presumed infections based on clinical and/or radiological findings. Acute GVHD (aGVHD) and thrombotic complications were assessed according to published consensus criteria [Citation19,Citation20]. NRM was defined as death without disease progression. The overall survival (OS) was defined as the time interval from the date of transplantation to the date of death or last follow-up. The event-free survival (EFS) was defined as the time from transplantation to treatment failure (death or relapse) or final follow-up.
Statistical analysis
Comparisons of patient characteristics between groups were performed with the χ2 test for categorical variables and Mann–Whitney test for continuous variables. The significance of the serum thyroid hormone concentrations was evaluated by receiver-operating characteristic (ROC) curve analysis, and the cut-off value was also determined based on ROC curves. Cumulative incidences were estimated for transplant-related complications (up to day 100) and NRM while considering relapse/death and relapse as competing events, respectively. The OS and EFS probabilities were estimated with the Kaplan–Meier method and compared with the log-rank test. Multivariate analyses for early NRM were performed with Cox proportional hazards regression. Multivariate models were constructed with stepwise forward selection using a p < 0.15 to include variables in the model. SPSS 17.0 was used for statistical analysis and p < 0.05 was considered significant. The endpoint of the last follow-up for all of survivors was 30 September 2016.
Results
Patient characteristics
Patients and treatment characteristics are summarized in . The median FT3 level before HCT was 5.1 pmol/l (range, 2.14–7.91). As discussed below, we set a cut-off FT3 level of 4.7 pmol/l to divide the patients into low- and high-FT3 groups (n = 144 and 330, respectively).
Table 1. Patient characteristics.
Transplantation outcomes
Neutrophil engraftment was achieved in 98% of patients, and 11 patients had disease progression or died of complications before achieving this endpoint. Of the patients who were evaluated for aGVHD, the cumulative incidence of grade II–IV aGVHD within 100 days was 36% [95% confidence interval (CI), 27–45%]. By day 100, the cumulative incidence of grade 5 infectious toxicities was 6% (95% CI, 3–8%). Thirteen patients experienced deadly thrombotic complications. The NRM was 11% at day 100. The median follow-up time for survivors was 41 months (range, 27–62 months). The 3-year NRM was 19%, the 3-year relapse rate was 13%, the 3-year OS was 67%, and the 3-year EFS was 65%.
Thyroid hormone concentrations
We performed ROC analyses and calculated the area under the curve (AUC) to evaluate the predictive value of the thyroid hormone concentrations for transplant-related complications. For the whole cohort, the baseline FT3 level lacked sufficient predictive power for predicting serious infection, aGVHD and thrombotic complications. However, the AUC of FT3 for early NRM within 100 days after sibling HCT was 0.73 (95% CI, 0.64–0.82), suggesting that the FT3 value could be used to predict early NRM in this setting (). The sensitivity and specificity were 68% and 73%, respectively, with a cut-off value of 4.7 pmol/l. On the other hand, the serum FT4 and TSH levels did not seem to be useful for predicting the NRM after allo-HCT, as the AUCs were close to 0.5.
Impact of the FT3 level on sibling transplantation outcomes
The cumulative incidences of early NRM within 100 days after sibling HCT were 14% (95% CI, 10–18%) in the low FT3 group and 6% (95% CI, 4–8%) in the high-FT3 group (p = 0.033, (a)). Due to the limited number of events to preclude statistical analysis, fatal infection, acute GVHD, vascular complications and bleeding may have all contributed to the increased early NRM in the low FT3 group. Moreover, the difference was also significant at 3 years after HCT (24% vs. 14%, p = 0.046, (b)), although NRM rates after day 100 were similar in the two groups. In univariate analysis, only recipient HCT-CI ≥ 3 and a baseline serum FT3 level <4.7 pmol/l were significantly associated with high early NRM. The lower FT3 level was significantly associated with a high early NRM in multivariate analysis (HR = 3.19, 95% CI, 1.13–9.03, p = 0.029, ). Additionally, recipients with lower FT3 levels had a trend towards inferior OS at 3 years after HCT [(66%, 95% CI, 60–72%) vs. (72%, 95% CI, 68–76%), p = 0.235, ], although the difference did not reach statistical significance.
Figure 2. Cumulative incidences of early NRM within 100 days (a) and NRM at 3 years (b) according to the baseline serum FT3 level.
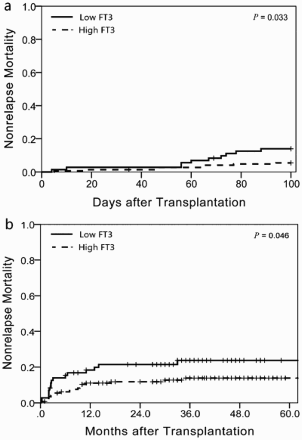
Table 2. Univariate and multivariate analysis of early NRM.
Discussion
In this retrospective study, we found a significant association between a low FT3 level prior to transplantation and the incidence of early NRM from HLA-matched donors. Our findings suggest that the FT3 level, but not the FT4 or TSH levels, at transplant can be used as a good biomarker for higher early NRM in patients undergoing sibling HCT.
Alterations in thyroid function tests instead of hypothyroidism are common in critical illness as well as chronic systemic diseases [Citation9–11], and it has been reported that a low serum concentration of FT3 is a strong independent predictor of unfavourable outcomes in many gravely ill patients [Citation15–17,Citation21]. A short-term decrease in thyroid hormone parameters could be observed in nearly all patients undergoing HCT and unrecovered thyroid abnormalities after transplantation were associated with a worse outcome [Citation22,Citation23]. However, there is limited information on the association between thyroid hormones before HCT and transplant outcomes. In the present study, we demonstrated that a low baseline FT3 level less than 4.7 pmol/l was significantly associated with the development of early NRM after sibling HCT, which may have contributed to a trend of inferior overall survival. FT3 is more sensitive to the impact of critical illness than FT4 and TSH concentrations [Citation24], which may explain why such a relationship was specifically observed for the serum FT3 level but not for the serum FT4 and TSH levels.
The possible mechanisms underlying the prognostic value of FT3 are probably multifactorial. The first possible explanation is the so-called non-thyroidal illness syndrome effect [Citation25]. Some data suggest that lower FT3 level represents the severity of the underlying disease and correlates with a subclinical unsatisfactory functional status of the body [Citation9–11,Citation24]. Therefore, it is likely that patients with a low FT3 may experience increased organ toxicities in response to myeloablative conditioning; moreover, there was a worse outcome after serious complication of HCT such as sepsis or septic shock, resulting in an increased NRM. Therefore, low FT3 might be used as an adjunctive sensitive parameter beyond HCT-CI for the HCT risk assessment because comorbidities were only found in a minority of patients undergoing ablative HCT [Citation5]. The other potential explanation is the mutual association between triiodothyronine and pro-inflammatory cytokines. Alterations in the circulating thyroid hormones have been demonstrated to inversely correlate with serum cytokine concentrations, such as IL-6 and TNF-α, in various medical conditions [Citation13,Citation14,Citation26,Citation27]. Based on the solid rationale that cytokines have a significant role in the development of aGVHD [Citation28], it is reasonable to expect that patients with a low baseline FT3 level will have a high probability for developing fatal aGVHD, which is the second cause of early NRM beyond infection [Citation29]. Moreover, studies have demonstrated that FT3 exerts direct and acute effects on enhancing endothelial function [Citation12,Citation30,Citation31], such that lower pretransplant serum FT3 levels are at a higher risk for subsequent microvascular occlusive disorders. Larger prospective clinical investigations to validate our results and give further insights into the mechanisms are warranted in the future. Meanwhile, further studies are also needed to investigate whether patients with a low FT3 level may benefit from thyroid hormone replacement therapy.
We only measured serum thyroid hormones before the initiation of conditioning regimens. Patients may experience different decline rates in their FT3 levels surrounding HCT, and Schulte et al. showed that the decrease in FT3 was more pronounced in those patients who eventually died after HCT [Citation23]. So, evaluating at 2 or 3 time points peritransplant to validate the predictive role of the magnitude of decline in FT3 from the day of conditioning initiation until its nadir after HCT is desirable.
In contrast with sibling HCT, we did not find a significant association between the baseline FT3 levels and incidence of NRM in matched unrelated donor and haplo-identical transplantation. The explanation for this discrepancy is not clear. We hypothesize that the conditioning regimen for patients who undergo transplantation from alternative donors which incorporates 10 mg/kg of antithymocyte globulin (ATG) as an in vivo T-cell depleting strategy to reduce the risk of acute GVHDs may contribute to the difference. Previous studies have suggested that patients transplanted using ATG are at a higher risk of opportunistic infection [Citation32]. As a result, the impact of ATG with an increased risk of serious infections, along with the inherently higher incidence of fatal aGVHD in unrelated or mismatched HCT [Citation33], might decrease the predictive value of FT3 levels, considering death resulting from infection and aGVHD accounts for the majority of early NRM. From the perspective of ATG, whether the results of this study could be extrapolated to sibling HCT with RIC regimens remains to be clarified in the future.
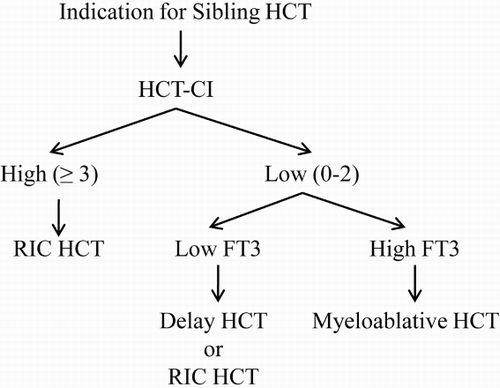
In summary, in this study we demonstrated that among patients undergoing myeloablative sibling transplantation, those displaying lower pretransplant serum FT3 levels are at higher risk for early NRM. We recommend integrating FT3 in composite risk models with HCT-CI for NRM prediction and HCT strategy selection to allow for individualized interventions according to the predicted risk ().
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496.
- Pollack SM, O'Connor TPJr, Hashash J, et al. Nonmyeloablative and reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation: a clinical review. Am J Clin Oncol. 2009;32(6):618–628.
- Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919.
- Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256.
- Sato M, Nakasone H, Oshima K, et al. Prediction of transplant-related complications by C-reactive protein levels before hematopoietic SCT. Bone Marrow Transplant. 2013;48(5):698–702.
- Ueda N, Chihara D, Kohno A, et al. Predictive value of circulating angiopoietin-2 for endothelial damage-related complications in allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1335–1340.
- Rashidi A, DiPersio JF, Westervelt P, et al. Peritransplant serum albumin decline predicts subsequent severe acute graft-versus-host disease after mucotoxic myeloablative conditioning. Biol Blood Marrow Transplant. 2016;22(6):1137–1141.
- Fliers E, Bianco AC, Langouche L, et al. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3(10):816–825.
- Iervasi G, Pingitore A, Landi P, et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107(5):708–713.
- Karadag F, Ozcan H, Karul AB, et al. Correlates of non-thyroidal illness syndrome in chronic obstructive pulmonary disease. Respir Med. 2007;101(7):1439–1446.
- Pingitore A, Landi P, Taddei MC, et al. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med. 2005;118(2):132–136.
- Rozing MP, Westendorp RG, Maier AB, et al. Serum triiodothyronine levels and inflammatory cytokine production capacity. Age (Dordr). 2012;34(1):195–201.
- Moura Neto A, Parisi MC, Alegre SM, et al. Relation of thyroid hormone abnormalities with subclinical inflammatory activity in patients with type 1 and type 2 diabetes mellitus. Endocrine. 2016;51(1):63–71.
- Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, et al. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol. 2011;164(2):147–155.
- Cerillo AG, Storti S, Kallushi E, et al. The low triiodothyronine syndrome: a strong predictor of low cardiac output and death in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2014;97(6):2089–2095.
- Rotondi M, Netti GS, Rosati A, et al. Pretransplant serum FT3 levels in kidney graft recipients are useful for identifying patients with higher risk for graft failure. Clin Endocrinol (Oxf). 2008;68(2):220–225.
- Wang Y, Chen F, Han Y, et al. Partially matched related hematopoietic stem cell transplantation without ex vivo T cell depletion compared with matched unrelated transplantation in adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2009;15(10):1258–1264.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
- Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–575.
- Suda S, Muraga K, Kanamaru T, et al. Low free triiodothyronine predicts poor functional outcome after acute ischemic stroke. J Neurol Sci. 2016;368:89–93.
- Vexiau P, Perez-Castiglioni P, Socié G, et al. The ‘euthyroid sick syndrome': incidence, risk factors and prognostic value soon after allogeneic bone marrow transplantation. Br J Haematol. 1993;85(4):778–782.
- Schulte C, Reinhardt W, Beelen D, et al. Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 1998;22(12):1171–1178.
- Moura Neto A, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol. 2016;2016(3):2157583.
- Farwell AP. Nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2013;20(5):478–484.
- Zoccali C, Tripepi G, Cutrupi S, et al. Low triiodothyronine: a new facet of inflammation in end-stage renal disease. J Am Soc Nephrol. 2005;16(9):2789–2795.
- Taki-Eldin A, Zhou L, Xie HY, et al. Triiodothyronine attenuates hepatic ischemia/reperfusion injury in a partial hepatectomy model through inhibition of proinflammatory cytokines, transcription factors, and adhesion molecules. J Surg Res. 2012;178(2):646–656.
- Henden AS, Hill GR. Cytokines in graft-versus-host disease. J Immunol. 2015;194(10):4604–4612.
- Teshima T, Reddy P, Zeiser R. Acute graft-versus-host disease: novel biological insights. Biol Blood Marrow Transplant. 2016;22(3 Suppl):11–16.
- Malyszko J, Malyszko JS, Pawlak K, et al. Possible relations between thyroid function, endothelium, and kidney and liver function in kidney allograft recipients. Transplant Proc. 2006;38(10):3509–3513.
- Videla LA, Fernández V, Cornejo P, et al. Thyroid hormone in the frontier of cell protection, survival and functional recovery. Expert Rev Mol Med. 2015;17:e10.
- Storek J, Mohty M, Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):959–970.
- Anasetti C. Use of alternative donors for allogeneic stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2015;2015(1):220–224.