ABSTRACT
Objective: To investigate the effect of thalidomide in patients with thalassemia intermedia.
Methods: We observed the effect of thalidomide in seven patients with thalassemia intermedia requiring blood transfusion. Four of the patients were transfusion-independent, and three patients were transfusion-dependent.
Results: For the four transfusion-independent patients, hemoglobin concentration increased significantly (≥2 g/dl) in three and moderately (1–2 g/dl) in one. After 3 months of treatment, hemoglobin concentration increased 3.2 ± 1.2 g/dl compared to pretreatment. Among the three transfusion-dependent patients, transfusion was terminated after one month of treatment in one patient and decreased >50% in the other two patients, accompanied by an increase in the average hemoglobin concentration.
Conclusion: Thalidomide had a significant effect in patients with thalassemia intermedia. Further studies of a larger scale and more rigorous design are warranted.
Thalassemia is the most common single gene hereditary disease worldwide. Depending on the gene involved, thalassemia is classified as either α thalassemia or β thalassemia. Beta thalassemia can be further divided into thalassemia major (TM) and thalassemia intermedia (TI) based on the severity of the disease [Citation1]. Due to a shortage of β globin, free α globin aggregates and takes part in the formation of reactive oxygen species, causing red blood cells (RBCs) to be destroyed in the bone marrow (ineffective erythropoiesis, IE) or peripheral circulation (hemolysis) [Citation2]. Different from patients with TM, TI patients can survive without regular blood transfusions and iron chelating, but multiple complications, such as pulmonary hypertension, extramedullary hemopoiesis, diabetes mellitus, leg ulcers and osteoporosis may occur due to anemia, IE or secondarily to iron overload [Citation3,Citation4]. Blood transfusion is effective in ameliorating IE, improving anemia and alleviating related complications [Citation5]. In a retrospective investigation of 165 patients with TI, 28% ultimately became transfusion-dependent in adulthood [Citation6]. However, iron overload will inevitably be aggravated after blood transfusion, and consequent iron chelating therapy is costly.
Guangxi province in the southwestern region of China is economically underdeveloped and an area with a high prevalence of thalassemia [Citation7,Citation8]. In Guangxi, there is a shortage of blood product supply, and many patients with thalassemia are not sufficiently transfused [Citation9]. Additionally, iron chelators are expensive and generally not affordable due to economic difficulties [Citation10]. It is important to investigate medicines that could possibly improve anemia in patients with TI in Guangxi. Hydroxyurea is the most commonly prescribed drug, and although it is effective in majority patients with TI [Citation11], its ability to improve anemia is moderate [Citation12]. In 2008, a dramatic effect of thalidomide in the treatment of one patient with TM was reported by Aguilar-Lopez et al. [Citation13]. Subsequently, four additional patients with β thalassemia were reported [Citation14–16], suggesting that thalidomide may be more effective than hydroxyurea in patients with thalassemia. Nevertheless, data is limited and publication bias cannot be ruled out, hence the effect of thalidomide on patients with TI remains to be explored. We investigated the effect of thalidomide in patients with TI who could not afford blood transfusion or chelation therapy in our center from May 2016, and data on seven patients is summarized below.
Patients and methods
Patients
From May 2016 on, adult patients with TI requiring blood transfusion but unable to afford regular transfusions or iron chelation due to economic or other reasons were recommended for thalidomide treatment. The indications for blood transfusion included the following: (1) continuous hemoglobin (Hb) concentration <7.0 g/dl, (2) complications such as extramedullary hematopoiesis, leg ulcers, facial deformities, and pulmonary hypertension, and (3) limited physical activities due to anemia [Citation17]. Patients were informed of the side effects and possible benefits of thalidomide treatment. Full informed consent was required before treatment initiated. Pregnancy was ruled out in female patients. All female patients were informed that pregnancy should be prevented during treatment and until 6 months after the withdrawal of medicine. The thalidomide protocol for patients with TI was approved by the Medical Ethics Committee of the 303rd People’s Liberation Army Hospital.
Drug administration and clinic observation
Patients were divided into two groups before treatment: the transfusion-independent (group 1) and transfusion-dependent group (group 2). Patients requiring blood transfusion every 1–2 months in the year before treatment were classified as transfusion-dependent. In group 1, no patients received blood transfusions within 3 months before or during treatment.
Thalidomide (Changzhou Pharmaceutical Factory, Changzhou, Jiangsu, China) was begun at 50 mg orally per night. A dosage adjustment was performed in one patient in Group 2. To prevent thrombosis, aspirin (100 mg/d) was prescribed for post-splenectomy patients who had platelet counts >500 × 109/l.
Red cell parameters, including Hb concentration, reticulocyte count, and nucleated red blood cell (NRBC) count, were analyzed using an XE 5000 automatic blood cell analyzer (Sysmex Corporation, Kobe, Japan). The levels of HbF and HbA were quantified using a BioRad Variant II high-pressure liquid chromatograph (BioRad, Hercules, CA, U.S.A.). Biochemical tests, including measurement of aspartate aminotransferase, alanine aminotransferase, bilirubin, and blood glucose, were assessed using a multichannel analyzer (Abbot Aeroset, Abbott Diagnostics, Bohemia, NY, U.S.A.).
Standard for efficacy judgment
For patients in group 1, Hb levels were measured after 3-month treatment and compared with pretreatment levels. A minor response was defined as an increment in the Hb concentration of 1–2 g/dl; the major response was Hb increment >2 g/dl. For patients in group 2, average Hb levels and blood transfusions 6 months before and after treatment were recorded. A minor response was defined as a 50% reduction in blood transfusions; the major response was defined as the need for a blood transfusion [Citation11,Citation18].
Statistical analysis
All data were analyzed using SPSS v13.0 (SPSS Inc., Chicago, IL, U.S.A.). For patients in group 1, a paired t-test was used to compare the changes in Hb concentration and other parameters before and after treatment. For patients in group 2, the mean Hb level and the difference in blood transfusion 6 months before and after treatment were compared using a two-sample t-test. P < 0.05 was considered statistically significant.
Results
Between May 2016 and February 2017, seven patients received thalidomide treatment. Among the seven patients, four were in group 1 and three were in group 2. At the time of the last follow-up (12 February 2017), patients had been treated for >3 months in group 1 and >6 months in group 2. The clinical data of the seven patients are shown in .
Table 1. Clinical characteristics of seven patients who received thalidomide.
Effect of thalidomide on patients in group 1
As shown in , for each patient in group 1, the Hb concentration increased after treatment with thalidomide. The Hb concentration increased after two weeks of treatment (1.0 ± 0.5 g/dl, P = 0.032), and continued to increase incrementally in three patients (except case 4).
Figure 1. Hemoglobin concentration after thalidomide treatment in four transfusion-independent patients with TI.
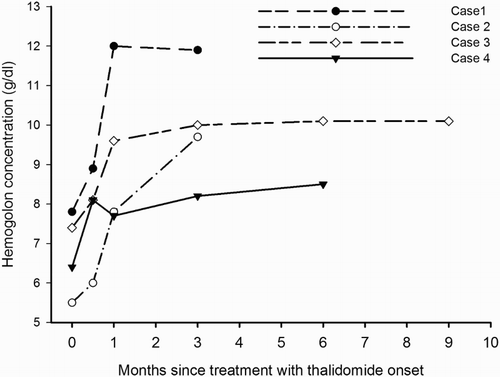
The hematologic and biochemical parameters of the four patients before and 3 months after treatment were compared. All four patients responded to treatment with thalidomide. Among the four patients, there were three major responses and one minor response. Hb concentration increased significantly after 3 months of treatment (3.2 ± 1.2 g/dl), while the reticulocyte and NRBC counts were not significantly changed. Hb analysis showed that the increased Hb was mainly composed of HbF; HbA was not significantly improved. Bilirubin was also not significantly changed ().
Table 2. Indices of anemia and hemolysis in group 2 patients before and after 3 months of treatment with thalidomide.
Effect of thalidomide on patients in group 2
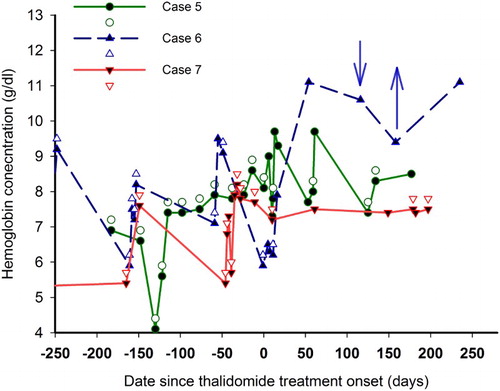
In case 6, hemoglobin concentration increased after 1 month of treatment, and the patients transformed from transfusion-dependent to transfusion-independent. From then on, Hb concentration remained at approximately 10 g/dl without transfusion. Her Hb concentration decreased at 4 months of treatment when the dosage of thalidomide decreased from 50 to 25 mg/d, but recovered when the dose of thalidomide increased.
In cases 5 and 7, the amount of blood transfusion and average Hb concentration 6 months before and after thalidomide treatment were recorded. After treatment, blood transfusion decreased from 13 units to 6 units in case 5, and from 23.5 units to 10.5 units in case 7. Simultaneously, average Hb concentration increased from 7.1 ± 1.3 g/dl to 8.5 ± 0.9 g/dl in case 5, and from 7.2 ± 1.3 g/dl to 8.4 ± 0.9 g/dl in case 7 ().
Figure 2. Effect of thalidomide in three patients with TI transfusion-dependent. Changes in Hb concentration and transfusions in different patient with TI are shown as lines and symbols with different colors. The solid symbols represent Hb concentration, and the open symbols represent blood transfusions. For case 6, ↓ indicates the decrease of thalidomide from 50 to 25 mg/d, and ↑ indicates the increase of thalidomide from 25 to 50 mg/d. After thalidomide treatment, the number of blood transfusions decreased, but Hb concentrations increased.
Side effects
Two patients complained of mild constipation and improved after diet modulation. No other side effects occurred during treatment. Dosage adjustments due to adverse reactions were not needed.
Discussion
Although the extant literature suggests a significant effect of thalidomide in patients with TI and TM, only five cases had been documented in four reports [Citation13–16]. The favorable effect of thalidomide was confirmed by the high response ratio (100%) in the seven patients treated in this study. Among the seven patients, four reached major response and three reached minor response. This suggests better efficacy than hydroxyurea monotherpay. Among 100 patients with TI studied by El-Beshlawy et al. [Citation11], the overall response ratio and major response ratios of hydroxyurea were 79 and 33%, respectively. In another report by Karimi et al. [Citation19], which consist of 106 patients TI, the overall response ratio and major response ratios of hydroxyurea were 47 and 3%, respectively. For patients with TI blood transfusion-dependent, the efficacy of hydroxyurea was further disappointing, with the overall response ratio and major response ratios as low as 30 and 0%, respectively [Citation20].
The effect of thalidomide seems to be comparable to regimens consisting of erythropoietin alone (overall response ratio 95% and major response ratio 67%) [Citation21] or in combination with hydroxyurea (overall response ratio 92.5% and major response ratio 50%) [Citation20]. However, thalidomide was more convenient and more economically feasible. For a 60-kg patient with TI, if blood transfusions are required every 3 months, the cost of thalidomide is about 1440 RMB Yuan (equal to 209 dollars) per year, while the yearly cost of erythropoietin is about 36,000 RMB Yuan, and the yearly cost of blood transfusion together with iron chelator is more than 40,000 RMB Yuan. It is also noteworthy that in the group 1 patients, Hb concentrations increased by an average of 3.2 g/dl after 3 months of treatment, which was much better than what would be expected from hydroxyurea (0.7–1.6 g/dl) [Citation11,Citation16,Citation22] or hydroxyurea combined with erythropoietin (1.6 g/dl) [Citation20].
To explore the possible mechanism underlying thalidomide in the treatment of patients with TI, changes in Hb composition were analyzed before and after treatment in group 1 patients. The increased Hb after treatment was mainly consisted of HbF, while HbA showed little change after treatment, suggesting that similar to hydroxyurea [Citation23], thalidomide improved anemia mainly by increasing the production of HbF in patients with TI [Citation13]. In vitro experiments have also demonstrated that thalidomide can induce expression of the gamma gene [Citation24–26]. We did not observe a decrease in the reticulocyte and NRBC count and bilirubin level, which reflect hemolytic activity during treatment. The former may be ascribed to the small number in this group, and the latter may be because bilirubin metabolism is also affected by the uridine diphosphate glucuronosy l transferase (UGT1A1) gene polymorphism, and thus does not accurately reflecting the degree of hemolysis [Citation27].
In conclusion, our observations suggest that thalidomide has a superior therapeutic effect in patients with TI. Thalidomide is convenient for oral administration and inexpensive in China. In this area, patients with TI may benefit from thalidomide treatment. Future more-standardized clinical trials are required to verify the efficacy and safety of thalidomide in treating patients with TI, to explore the suitable dosage for treatment, and the possible index for predicating responses.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Xiaolin Yin http://orcid.org/0000-0003-0500-189X
Additional information
Funding
References
- Origa R. β-Thalassemia. Genet Med. 2016. DOI:10.1038/gim.2016.173
- Padate B, Bain BJ, de la Fuente J. Ineffective hemopoietic in beta thalassemia major visualised. Am J Hematol. 2011;86(4):372. doi: 10.1002/ajh.21953
- Taher AT, Musallam KM, Karimi M, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886–1892. doi: 10.1182/blood-2009-09-243154
- Vichinsky E. Non-transfusion-dependent thalassemia and thalassemia intermedia: epidemiology, complications, and management. Curr Med Res Opin. 2016;32(1):191–204. doi: 10.1185/03007995.2015.1110128
- Taher A, Vichinsky E, Musallam K, et al. Guidelines for the management of non transfusion dependent thalassaemia (NTDT). Nicosia: Thalassaemia International Federation;2013.
- Camaschella C, Cappellini MD. Thalassemia intermedia. Haematologica. 1995;80(1):58–68.
- Chen W, Zhang X, Shang X, et al. The molecular basis of beta-thalassemia intermedia in southern China: genotypic heterogeneity and phenotypic diversity. BMC Med Genet. 2010;13:31.
- Xiong F, Sun M, Zhang X, et al. Molecular epidemiological survey of haemoglobinopathies in the Guangxi Zhuang Autonomous Region of southern China. Clin Genet. 2010;78(2):139–148. doi: 10.1111/j.1399-0004.2010.01430.x
- Yin XL, Wu ZK, He YY, et al. Treatment and complications of thalassemia major in Guangxi, Southern China. Pediatr Blood Cancer. 2011;57(7):1174–1178. doi: 10.1002/pbc.23101
- Xu LH, Fang JP. The current status of β-thalassemia major in Mainland China. Hemoglobin. 2013;37(4):307–314. doi: 10.3109/03630269.2013.789967
- El-Beshlawy A, El-Ghamrawy M, EL-Ela MA, et al. Response to hydroxycarbamide in pediatric β-thalassemia intermedia: 8 years’ follow-up in Egypt. Ann Hematol. 2014;93(12):2045–2050. doi: 10.1007/s00277-014-2154-5
- Kosaryan M, Zafari M, Alipur A, et al. The effect and side effect of hydroxyurea therapy on patients with β-thalassemia: a systematic review to December 2012. Hemoglobin. 2014;38(4):262–271. doi: 10.3109/03630269.2014.927770
- Aguilar-Lopez LB, Delgado-Lamas JL, Rubio-Jurado B, et al. Thalidomide therapy in a patient with thalassemia major. Blood Cells Mol Dis. 2008;41(1):136–137. doi: 10.1016/j.bcmd.2008.03.001
- Fozza C, Pardini S, Giannico DB, et al. Dramatic erythroid response to low-dose thalidomide in two patients with transfusion independent thalassemia and severe post-transfusional alloimmune hemolysis. Am J Hematol. 2015;90(7):E141–E141. doi: 10.1002/ajh.24030
- Ricchi P, Costantini S, Spasiano A, et al. The long-term and extensive efficacy of low dose thalidomide in a case of an untransfusable patient with non-transfusion-dependent thalassemia. Blood Cells Mol Dis. 2016;57:97–99. doi: 10.1016/j.bcmd.2016.01.003
- Masera N, Tavecchia L, Capra M, et al. Optimal response to thalidomide in a patient with thalassaemia major resistant to conventional therapy. Blood Transfus. 2010;8(1):63–65.
- Taher AT, Musallam KM, Cappellini MD, et al. Optimal management of β thalassaemia intermedia. Br J Haematol. 2011;152(5):512–523. doi: 10.1111/j.1365-2141.2010.08486.x
- Olivieri NF, Saunthararajah Y, Thayalasuthan V, et al. A pilot study of subcutaneous decitabine in beta-thalassemia intermedia. Blood. 2011;118(10):2708–2711. doi: 10.1182/blood-2011-03-341909
- Karimi M, Haghpanah S, Farhadi A, et al. Genotype-phenotype relationship of patients with β-thalassemia taking hydroxyurea: a 13-year experience in Iran. Int J Hematol. 2012;95(1):51–56. doi: 10.1007/s12185-011-0985-6
- Elalfy MS, Adly AA, Ismail EA, et al. Therapeutic superiority and safety of combined hydroxyurea with recombinant human erythropoietin over hydroxyurea in young β-thalassemia intermedia patients. Eur J Haematol. 2013;91(6):522–533. doi: 10.1111/ejh.12182
- Ch A, Alimirzoyeva Z, Hasanova M, et al. Clinical application of recombinant erythropoietin in beta-thalassaemia intermedia. Georgian Med News. 2016;255(6):86–92.
- Karimi M, Zarei T, Haghpanah S, et al. Relationship between some single-nucleotide polymorphism and response to hydroxyurea therapy in Iranian patients with ß-thalassemia intermedia. J Pediatr Hematol Oncol. 2017;39(4):e171–e176. doi: 10.1097/MPH.0000000000000779
- Ehsani MA, Hedayati-Asl AA, Bagheri A, et al. Hydroxyurea-induced hematological response in transfusion-independent beta-thalassemia intermedia: case series and review of literature. Pediatr Hematol Oncol. 2009;26(8):560–565. doi: 10.3109/08880010903271671
- Fard AD, Kaviani S, Noruzinia M, et al. Evaluation of H3 histone methylation and colony formation in erythroid progenitors treated with thalidomide and sodium butyrate. Lab Hematol. 2013;19(1):1–5. doi: 10.1532/LH96.12003
- Aerbajinai W, Zhu J, Gao Z, et al. Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood. 2007;110(8):2864–2871. doi: 10.1182/blood-2007-01-065201
- Jalali FMA, Dehghani FA, Hajizamani S, et al. Thalidomide is more efficient than sodium butyrate in enhancing GATA-1 and EKLF gene expression in erythroid progenitors derived from HSCs with β-globin gene mutation. Int J Hematol Oncol Stem Cell Res. 2016;10(1):37–41.
- Italia KY, Jijina FF, Jain D, et al. The effect of UGT1A1 promoter polymorphism on bilirubin response to hydroxyurea therapy in hemoglobinopathies. Clin Biochem. 2010;43(16–17):1329–1332. doi: 10.1016/j.clinbiochem.2010.08.006
