ABSTRACT
Objectives: Immunophenotype is an important prognostic factor for childhood and adult T-cell acute lymphoblastic leukemia. However, immunophenotypic data from adult patients with T-cell lymphoblastic lymphoma (T-LBL) are scarcely available.
Methods: Subjects were unselected adult patients with T-LBL who were treated with intensive chemotherapy. Immunophenotyping of tumor cells was performed according to standard techniques.
Results: A total of eight patients with a median age of 31 years were analyzed who received hyper-CVAD treatment for LBL. Immunophenotypic analysis showed that the most common tumor type was cortical T-cell type [early T (n = 2), cortical T (n = 4), and medullary T (n = 2)]. Two patients diagnosed with early T-cell type had early disease progression.
Conclusions: Assessment of T-cell differentiation stages in malignant T lymphoblasts would be important in choosing treatment strategies for adult patients with T-LBL.
Introduction
T-cell lymphoblastic lymphoma (T-LBL) is a neoplasm of lymphoblasts committed to the T-cell lineage. The cell of origin is the precursor T-cell blasts whose differentiation is arrested at discrete stages of maturation [Citation1,Citation2]. Cell-marker profiling has been well studied in pediatric acute lymphoblastic leukemia (ALL). Early T-cell precursor ALL (ETP-ALL) has been identified as a high-risk subgroup of T-cell ALL (T-ALL) [Citation1,Citation3]. However, there are less data on immunophenotypic analysis confined only to adult patients with T-LBL, a disease entity similar to ALL. Here, immunophenotypic analysis was performed to evaluate the clinical impact of T-cell differentiation stages in adult patients with T-LBL.
Patients and methods
Patients and samples
This study was approved by our institutional review board. Subjects were unselected adult patients with T-LBL who were treated with intensive chemotherapy at Aichi Cancer Center Hospital from March 2005 to October 2015. Patients were diagnosed with T-LBL based on the presence of mediastinal masses and nodal lesions, regardless of whether there were 25% or more blasts in the bone marrow. All patients received hyper-CVAD alternating with high-dose methotrexate and cytarabine, followed by maintenance and intensification [Citation4]. Dosage adjustments were made based on each physician’s judgment. Patients with mediastinal disease at presentation were scheduled to receive consolidative irradiation. None of the patient underwent hematopoietic stem cell transplantation.
Flow cytometric analysis and immunohistochemical staining
Surface immunophenotyping of tumor cells was performed according to standard techniques that we have previously described [Citation5]. Immunophenotypic detection was mainly performed by three- and six-color flow cytometry. Immunohistochemistry was used to supplement diagnosis. Stages of T-cell differentiation were defined by the current World Health Organization (WHO) classification (Supplementary Table 1). Lymphoid-associated antibodies to the following cell surface markers were used: CD1a, CD2, cytoplasmic CD3, surface CD3, CD4, CD5, CD7, CD8, CD34, terminal deoxynucleotidyl transferase (TdT), and CD56. Early T-cell precursor (ETP) markers were examined using immunophenotypic techniques available for routine diagnosis. The diagnosis of early T-cell precursor lymphoblastic lymphoma (ETP-LBL) was made when the T lymphoblast-phenotype was CD1a−, CD8−, and CD5dim, in conjunction with expression of at least one of the following myeloid or stem cell antigens: CD13, CD33, CD34, CD117, or HLA-DR [Citation1,Citation3].
Results
Characteristics of patients
A total of eight patients were analyzed. The median age was 31 years (range: 18–68 years). Six patients were diagnosed with stage IV disease. Detailed patient characteristics are listed in . All patients expressed cytoplasmic CD3, CD5, CD7, and TdT. CD1a was positive in four cases and two cases showed dual positivity for CD4 and CD8. Immunophenotypic analysis of tumor cells led to the classification of two cases as medullary T-cell phenotype (UPN 2 and 5), four as cortical T-cell phenotype (UPN 1, 3, 4, and 8), one as pre-T-cell phenotype (UPN 7), and one as pro-T-cell phenotype (UPN 6) (). The patient with the pro-T-cell phenotype was diagnosed as ETP-LBL ().
Figure 1. Immunophenotyping of ETP. Tumor samples from a patient (UPN 6) before induction chemotherapy showed ETP phenotype of CD1a−, CD8−, and CD5dim, in conjunction with expression of stem cell antigen CD34.
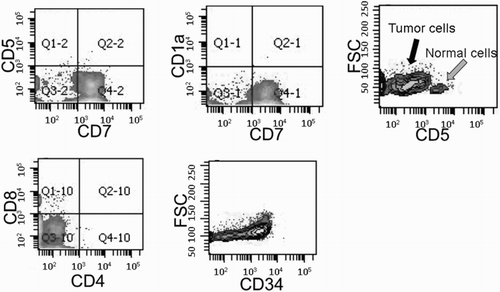
Table 1. Patients’ characteristics.
Table 2. Immunophenotypic analysis of tumor cells.
Clinical characteristics of patients with progressive disease: identification of early T-cell precursor phenotype
Over a median follow-up of 40 months (range: 10–93 months), two patients experienced treatment failure, whereas the remaining six were still in remission. The two patients with treatment failure had pro-T and pre-T-cell immunophenotypes (UPN 6 and 7). The tumor cells were positive for CD56 and CD99 and negative for CD57. The patient with ETP-LBL (UPN 6) had disease progression during intensive chemotherapy. Despite the administration of salvage chemotherapy, the patient died of lymphoma 10 months after initial diagnosis. The other patient, an elderly man (UPN 7) with the pre-T-cell phenotype, had a complete radiological remission after three cycles of the regimen. However, therapy was discontinued after four cycles owing to toxicity and poor performance status. We chose irradiation as a consolidation therapy, but as radiation therapy was being planned, rapid progression was observed at the site of the original tumor. After that, he underwent salvage chemoradiotherapy [Citation6]. Although in complete remission after salvage therapy, the patient died of a secondary myelodysplastic syndrome.
Discussion
We analyzed eight consecutive LBL patients who received hyper-CVAD treatment. Immunophenotypic analysis showed that the most common tumor type was cortical T-cell type. Expression of CD4 and CD8, or CD4/CD8 double positive cases were less frequently observed compared to those in a Children’s Oncology Group [Citation7]. Two cases were diagnosed as medullary T-cell phenotype, four as cortical T-cell phenotype, one as pre-T-cell phenotype, and one as pro-T-cell phenotype. The patient with the pro-T-cell phenotype was diagnosed as ETP-LBL.
ETPs are considered to be a subset of early thymic immigrants from the bone marrow and have multi-lineage differentiation potential [Citation1]. ETP-ALL/LBL accounts for 5–21% of all T-ALL/LBL in children and 13–32% in children, adolescents, and adults (). According to different populations, ETP-ALL/LBL accounts for 5% in Japan and for 11–32% in Europe or America () [Citation3,Citation7–21]. Researchers at a pediatric institution analyzed T-ALL patients using microarray and identified cases with ETP features [Citation3]. In their study, ETP-ALL had a poor prognosis compared with that of typical ALL. ETP-ALL is well characterized in pediatric cases; however, immunophenotyping in adult T-LBL has not been as well reported. Here, we analyzed the presenting features of adult patients with T-LBL. T-LBL was stratified into stages of T-cell differentiation as defined by the current WHO classification. Of the eight cases, one tumor with the pro-T-cell phenotype was diagnosed as ETP-LBL (12.5% of all cases). The patient experienced early disease progression during induction therapy. As is the case with ALL, ETP-LBL may represent a high-risk disease subtype in adult patients with LBL. Although our study included a relatively small number of patients, our findings are in line with a recent report from the MD Anderson Cancer Center analyzing clinical outcomes of adults with T-ALL/T-LBL [Citation20].
Table 3. Treatment results according to immunophenotype data on T-ALL/LBL.
Although ETP-ALL responds poorly to chemotherapy and has a very high risk of relapse in children and adults [Citation3,Citation11,Citation19], there are some contrary reports [Citation12,Citation13,Citation15]. There are remarkable little data published on whether or how the immunophenotype of adult LBL differs from childhood T-LBL/ALL. shows treatment results according to immunophenotype data on T-ALL/LBL. In children and adolescents, pro/pre-T-cell and immature immunophenotypes were not associated with worse survival [Citation8–10]; on the other hand, the phenotypes of ETP-ALL/LBL were associated with worse survival in two of the five studies (). In patients with adult ETP-ALL/LBL, about half of the study suggested that both immature and ETP phenotype could affect worse outcomes (). The prognostic value of immunophenotyping is still under investigation, especially in adult patients with T-LBL. Recent advances in molecular studies could lead to selection of optimal treatments for individuals with ETP-ALL [Citation23]. Comprehensive studies including a focus on genetic alterations as well as immunophenotypic analysis are required for better understanding of the characteristics of ETP-LBL.
T-ALL and T-LBL are defined as the same entity, i.e. precursor lymphoid neoplasms, in the current WHO classification. Although there are several studies defining genetic and molecular differences between T-ALL and T-LBL, the two diseases are usually separated by an arbitrary cut point of 25% bone marrow infiltration. Bone marrow infiltration below 25% is considered to be T-LBL. The degree of blast infiltration has, therefore, been used as the sole criterion to distinguish between T-ALL and T-LBL in many clinical trials. In this study, two patients with ≥25% bone marrow blasts were treated as having T-LBL (UPN 1 and 3), both with cortical T-cell phenotypes. One patient (UPN 1) had a mediastinal mass and received consolidative mediastinal irradiation, whereas the other presented with cervical and axillary lymphadenopathy. Both patients have remained in complete remission. Because adult ALL is generally considered to have an unfavorable prognosis, the use of T-LBL-specific therapies may be a rational approach for some patients who are diagnosed with T-ALL.
In summary, our study yielded two suggestive findings: (1) ETP-LBL defined by immunophenotyping had a poor prognosis and (2) T-LBL-specific therapies may be a rational treatment approach for some patients diagnosed with T-ALL in clinical trials. Further studies are needed to validate the results of our analysis in a large cohort of cases.
Supplementary data.xlsx
Download MS Excel (10.7 KB)Acknowledgements
The authors thank Yasutaka Okada, Koichi Koike, and Mako Hagino for their excellent laboratory works; and Daiki Hirano for data collection. This study was presented at the 74th Annual Meeting of the Japanese Cancer Association, 8–10 October 2015.
Disclosure statement
K.Y. has received honoraria from Kyowa Hakko Kirin. T.K. has received honoraria from Eli Lilly and Kyowa Hakko Kirin.
Additional information
Funding
References
- Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840
- Burkhardt B. Paediatric lymphoblastic T-cell leukaemia and lymphoma: one or two diseases? Br J Haematol. 2010;149:653–668. doi: 10.1111/j.1365-2141.2009.08006.x
- Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0
- Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi: 10.1182/blood-2003-12-4428
- Kato H, Yamamoto K, Oki Y, et al. Clinical value of flow cytometric immunophenotypic analysis for minimal residual disease detection in autologous stem-cell products of follicular and mantle cell lymphomas. Leukemia. 2012;26:166–169. doi: 10.1038/leu.2011.183
- Hirano D, Kato H, Kodaira T, et al. Salvage therapy with single agent L-asparaginase followed by local irradiation in an elderly patient with CD56-positve primary isolated extramedullary T-cell lymphoblastic lymphoma of the sinus. Ann Hematol. 2015;94:173–175. doi: 10.1007/s00277-014-2128-7
- Patel JL, Smith LM, Anderson J, et al. The immunophenotype of T-lymphoblastic lymphoma in children and adolescents: a Children’s Oncology Group report. Br J Haematol. 2012;159:454–461. doi: 10.1111/bjh.12042
- Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000;95:416–421.
- van Grotel M, Meijerink JP, van Wering ER, et al. Prognostic significance of molecular-cytogenetic abnormalities in pediatric T-ALL is not explained by immunophenotypic differences. Leukemia. 2008;22:124–131. doi: 10.1038/sj.leu.2404957
- Kobayashi R, Takimoto T, Nakazawa A, et al. Inferior outcomes of stage III T lymphoblastic lymphoma relative to stage IV lymphoma and T-acute lymphoblastic leukemia: long-term comparison of outcomes in the JACLS NHL T-98 and ALL T-97 protocols. Int J Hematol. 2014;99:743–749. doi: 10.1007/s12185-014-1585-z
- Inukai T, Kiyokawa N, Campana D, et al. Clinical significance of early T-cell precursor acute lymphoblastic leukaemia: results of the Tokyo Children’s Cancer Study Group Study L99-15. Br J Haematol. 2012;156:358–365. doi: 10.1111/j.1365-2141.2011.08955.x
- Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014;166:421–424. doi: 10.1111/bjh.12882
- Madanat F, Jaber H, Azayyat I, et al. Features and outcomes of pediatric early T cell leukemia: King Hussein Cancer Center experience. Hematol Oncol Stem Cell Ther. 2016;9:126–128. doi: 10.1016/j.hemonc.2015.09.001
- Vitale A, Guarini A, Ariola C, et al. Adult T-cell acute lymphoblastic leukemia: biologic profile at presentation and correlation with response to induction treatment in patients enrolled in the GIMEMA LAL 0496 protocol. Blood. 2006;107:473–479. doi: 10.1182/blood-2005-04-1754
- Neumann M, Heesch S, Gokbuget N, et al. Clinical and molecular characterization of early T-cell precursor leukemia: a high-risk subgroup in adult T-ALL with a high frequency of FLT3 mutations. Blood Cancer J. 2012;2:e55. doi: 10.1038/bcj.2011.49
- Van Vlierberghe P, Ambesi-Impiombato A, De Keersmaecker K, et al. Prognostic relevance of integrated genetic profiling in adult T-cell acute lymphoblastic leukemia. Blood. 2013;122:74–82. doi: 10.1182/blood-2013-03-491092
- Allen A, Sireci A, Colovai A, et al. Early T-cell precursor leukemia/lymphoma in adults and children. Leuk Res. 2013;37:1027–1034. doi: 10.1016/j.leukres.2013.06.010
- Shimizu H, Handa H, Hatsumi N, et al. Distinctive disease subgroups according to differentiation stages in adult patients with T-cell acute lymphoblastic leukemia. Eur J Haematol. 2013;90:301–307. doi: 10.1111/ejh.12088
- Chopra A, Bakhshi S, Pramanik SK, et al. Immunophenotypic analysis of T-acute lymphoblastic leukemia. A CD5-based ETP-ALL perspective of non-ETP T-ALL. Eur J Haematol. 2014;92:211–218. doi: 10.1111/ejh.12238
- Jain N, Lamb AV, O’Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127:1863–1869. doi: 10.1182/blood-2015-08-661702
- Brammer JE, Saliba RM, Jorgensen JL, et al. Multi-center analysis of the effect of T-cell acute lymphoblastic leukemia subtype and minimal residual disease on allogeneic stem cell transplantation outcomes. Bone Marrow Transplant. 2017;52:20–27. doi: 10.1038/bmt.2016.194
- Guo RJ, Bahmanyar M, Minden MD, et al. CD33, not early precursor T-cell phenotype, is associated with adverse outcome in adult T-cell acute lymphoblastic leukaemia. Br J Haematol. 2015;172:823–825. doi: 10.1111/bjh.13545
- Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725
