ABSTRACT
Background: Patients with cancer commonly demonstrate laboratory evidence for hypercoagulability. Coagulation and inflammation play a role in the pathophysiology of hematological malignancies and the correlation between hypercoagulability and inflammation with tumor outcomes and the patient’s prognosis are well studied.
Objective: To identify an association between hemostasis activation, fibrinolysis and inflammation with mortality in patients with hematological malignancies to determine their prognostic significance.
Methods: This study is a prospective observational cohort study; Hypercoagulability and inflammatory biomarkers including:(1) Coagulation and fibrinolysis activation Markers (D-dimer, Fibrinogen, Antithrombin, plasminogen activator inhibitor 1 [PAI-1]);(2) Endothelium and platelet activation Markers (von Willebrand Factor [vWF], soluble P-selectin); and (3) Inflammation Markers (Tumor necrosis factor alpha [TNF-α], Interleukin-6 [IL-6]) were assayed on a group of 171 patients with hematological malignancies at time of diagnosis. They have been followed up for an average period of 416.8 days with an endpoint of mortality.
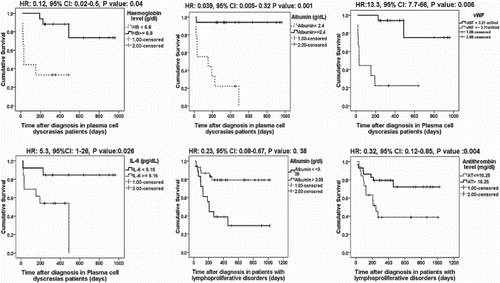
Results: Sixty patients died during follow up. There were statistically significant associations between Plasma cell dyscrasias mortality and ECOG performance status (P value:<0.005), Hemoglobin level (P value: 0.04), serum Albumin level (P value: 0.001), vWF (P value: 0.006) and IL-6 (P value 0.015), and between lymphoproliferative disorders mortality and presence of B symptoms (P value: 0.02), ECOG performance status (P value:<0.02), serum Albumin level (P value: 0.038), Antithrombin (P value: 0.004).
Conclusion: Some biomarkers of coagulation and inflammation showed statistically significant associations with plasma cell dyscrasias mortality (vWF and IL-6) and lymphoproliferative disorders mortality (Antithrombin) and potentially could be used as prognostic markers.
Introduction
The relationship between cancer and venous thromboembolism (VTE) has been recognized for many years and Armand Trousseau was the first to describe a clinical association between thrombosis and undiagnosed cancer over 100 years ago [Citation1].
Patients with cancer commonly demonstrate laboratory evidence for subclinical hypercoagulability even in the absence of overt clinical thrombosis. Consideration of the pathophysiology of this hypercoagulability is important to the design of appropriate intervention measures either to prevent or treat thrombohemorrhagic complications of the hematologic malignancies [Citation2].
The pathogenesis of hypercoagulability in cancer is complex and multifactorial. Multiple hemostatic derangements are proved to exist with malignancies, as high levels of plasma by-products of clotting reactions (i.e. prothrombin fragment 1+2 [F1+2], D-dimer, fibrinopeptide A, and thrombin–antithrombin complex), or high levels of circulating microparticles produced by tumor cells and platelets as well as an acquired protein C resistance [Citation3]. On the other hand, hemostatic proteins and reactions play a role in the process of angiogenesis, tumor cell invasion, tumor progression, and metastatic spread [Citation4].
In the last decade, many studies looked at the role of hypercoagulability and inflammation in the pathophysiology of hematological malignancies and the extent of their correlation with tumor stage, outcomes, and the patient’s prognosis [Citation5–9].
This study was a prospective observational cohort study aiming to identify an association between the activation of hemostasis and fibrinolysis as well as inflammation with mortality in patients with hematological malignancies to determine their role in prognosis.
Patients and methods
The study was conducted on a group of 171 patients with malignant hematological conditions treated in the hematology unit in Cairo University hospital between 2013 and 2015. Adult patients with newly diagnosed hematologic malignancies or relapse of disease after complete or partial remission were considered for inclusion in the study. Exclusion criteria were overt bacterial or viral infection within the previous 2 weeks, venous or arterial thromboembolism within the previous 3 months, and anticoagulation with vitamin K antagonists or low molecular weight heparin (LMWH). Patients were allowed to take antiplatelet agents and treated with LMWH as thromboprophylaxis [during their hospital stay or myeloma patients on immunomodulatory drugs (IMiDs)], following surgery or radiotherapy within the previous 2 weeks and chemotherapy within the previous 3 months to exclude a transient influence of these interventions on the hemostatic system.
Laboratory and/or histological testing confirmation of the diagnosis of hematological malignancy were a prerequisite. Hypercoagulability was assessed at entry into the study by measuring the circulating levels of the following parameters: (1) Markers of coagulation and fibrinolysis activation [D-dimer, fibrinogen, antithrombin, plasminogen activator inhibitor 1 (PAI-1)]; (2) Markers of endothelium and platelet activation [von Willebrand Factor (vWF), soluble P-selectin]; and (3) Markers of inflammation [Tumor necrosis factor alpha (TNF-α), Interleukin-6 (IL-6)]. End point was mortality.
Ten milliliters of blood (drawn directly by venipuncture) were collected from each subject and divided into two sterile citrate vaccutainers (for fibrinogen, AT III, P-selectin, vWF, PAI-1 antigen, and D-Dimer) and one sterile Ethylenediamin tetraacetic acid (EDTA) vaccutainer (for IL-6 and TNF-α).
Samples were centrifuged immediately at 4000 RPM for 15 minutes and plasma was separated into aliquots and stored at −20°C then brought to room temperature prior to the assay. Hemolysed or clotted samples were discarded.
Commercial ELISA methods were used to measure plasma levels of D-dimer (Technozym®, Technoclone, Vienna, Austria), PAI-1, VWF antigen, TNF-a, and IL-6 (all from AssayMax®, AssayPro, St. Charles, MO, U.S.A.), human fibrinogen (GenWay, San Diego, CA, U.S.A.), human pselectin (RayBio, Norcross, GA, U.S.A.). The ELISA biomarkers were performed according to the manufacturer’s instructions. Antithrombin analysis was performed by Radial Immunodiffusion in the range of 15–20 mg/dl (Haemtech, Essex Junction, VT, U.S.A.). PT was performed using neoplastine reagent (Stago Coagulation AQ6analyzer, Stago, Parsippany, NJ, U.S.A.). All analyses were performed within 5 months from blood collection in a blinded fashion in the central laboratory of Kasr Alainy Faculty of Medicine, Cairo University, Egypt.
Statistical methods used were as follows: Statistical package for social science program version 16.0 was used for the analysis of data. Data were summarized as mean, standard deviation (SD). Independent samples’ t-test was used for parametric quantitative variables, Mann–Whitney test was used for the analysis of non-parametric quantitative data. Chi square was used for the analysis of qualitative data. Receiver operating characteristic (ROC) curve was used for determining cut-off values for laboratory biomarkers. Kaplan–Meier method and Cox proportional hazard model were used for survival analysis. p-value was considered significant if <0.05.
Results
Patient demographics
The study included 171 patients with malignant hematological conditions followed up for an average period of 416.82 days with an endpoint of mortality. The mean age was 48.6 years with 79 (46.2%) female patients and 92 (53.8%) male patients. Out of 171 patients, 48 (28.1%) were diagnosed to have lymphoproliferative disorders (7 had Hodgkin lymphoma, 14 had follicular lymphoma, 9 had diffuse large B cell lymphoma, 12 had chronic lymphocytic leukemia, 3 had hairy cell leukemia, 2 had T-cell lymphoma, and 1 had Burkitt lymphoma, 40 (23.4%) myeloproliferative neoplasms (26 had chronic myeloid leukemia, 6 had polythycemia vera, 3 had essential thrombocythemia, and 5 had primary myelofibrosis), 38 (22.2%) were diagnosed to have acute myeloid leukemia (AML) (35) (or myelodysplastic syndrome progressed to AML(3)), 19 (11.1%) were diagnosed to have acute lymphoblastic leukemia (ALL) (all are B-ALL), 26 (15.2%) were diagnosed to have plasma cell dyscrasias (21 had myeloma, 2 had AL amyloidosis, 3 had Waldenström’s macroglobulinemia). All patients were newly diagnosed except six had relapsed disease (two with AML, two with ALL, and two with Follicular lymphoma).
One hundred and ten (64.3%) patients had one or more of constitutional manifestations (fever, weight loss, excessive sweating), 77 (45%) patients had one or more co-morbidities including diabetes mellitus, hypertension, ischemic heart disease, renal impairment, liver cell failure, tuberculosis, psoriasis, Parkinsonism, Guillian–Barre, and hypothyroidism. Eastern Cooperative Oncology Group (ECOG) performance status showed a mean of 2.11, SD of 1.087, Body mass index (BMI) showed a mean of 26.14 kgm/m2, and SD of 5.14. 115 (67.3%) patients had organomegaly (hepatomegaly and/or splenomegaly), 51 patients (29.8%) had lymphadenopathy. Thirteen (7.6%) patients underwent a surgical procedure (all were lymph node biopsies), 28 (16.4%) had a central venous access device CVAD inserted, 156 (91.2%) patients received chemotherapy, two (1.2%) patients received radiotherapy, two patients received IMIDs for myeloma, and eight (4.7%) received erythropoiesis-stimulating agents.
Mortality and laboratory biomarkers
Sixty (35.1%) patients died during follow-up. Forty-five (75%) patients died within 6 months after diagnosis, 11 (18.3%) patients died within 12 months, and 4 (6.7%) died within 18 months.
Out of 60 patients who died during follow-up, 34 (56.7%) died of disease progression, 17 (28.3%) died of infection, 3 (5%) died of pulmonary embolism (diagnosed by CT pulmonary angiography), 3 (5%) died of hepatic failure, 2 (3.3%) died of heart failure, and 1 (1.7%) died of intracranial bleeding (diagnosed by CT head).
There were statistically significant associations between plasma cell dyscrasias mortality and ECOG performance status (p value: <0.005), hemoglobin level (p value: 0.04), serum albumin level (p value: 0.001), vWF (p value: 0.006), and IL-6 (p value 0.015) ().
Table 1. Mortality and laboratory biomarkers.
For prediction of plasma cell dyscrasias mortality, ROC curve of hemoglobin showed that a level of 6.8 g/dl showed the highest likelihood ratio (LR) of 3.78 with a sensitivity of 66.7% and a specificity of 87%. ROC curve of albumin level showed that a level of 2.4 g/dl showed the highest LR of 14.3 with a sensitivity of 88.9% and a specificity of 99%. ROC curve of vWF showed that a level of 3.31 mU/ml showed the highest LR of 6.95 with a sensitivity of 77.8% and a specificity of 88.2%. ROC curve of IL-6 levels showed that a level of 5.15 pg/ml showed the highest LR of 2.2 with a sensitivity of 77.8% and a specificity of 65% ().
There were statistically significant associations between mortality of patients with lymphoproliferative disorders and the presence of B symptoms (p value: 0.02), ECOG performance status (p value: <0.02), serum albumin level (p value: 0.038), antithrombin (p value: 0.004). For prediction of lymphoproliferative disorders mortality, ROC curve of albumin level showed that a level of 3.04 g/dl showed the highest LR of 3.2 with a sensitivity of 61% and a specificity of 73.7%. ROC curve of antithrombin showed that a level of 16.25 mg/dl showed the highest LR of 2.28 with a sensitivity of 66.7% and a specificity of 79.3% ().
There were statistically significant associations between mortality of patients with myeloproliferative disorders and older age (p value: 0.039), ECOG performance status (p value: <0.001).
There were statistically significant associations between mortality of patients with ALL and ECOG performance status (p value: <0.02), PTT (p value: 0.005).
There have been no statistically significant associations between mortality of patients with AML and any of the tested parameters.
Discussion
Our study showed statistically significant associations between plasma cell dyscrasias mortality and ECOG performance status (p value: <0.005), hemoglobin level (p value: 0.04), serum albumin level (p value: 0.001), vWF (p value: 0.006), and IL-6 (p value 0.015), and between mortality of patients with lymphoproliferative disorders and the presence of B symptoms (p value: 0.02), ECOG performance status (p value: <0.02), serum albumin level (p value: 0.038), antithrombin (p value: 0.004).
In hematological diseases, TNF-α has been shown to be a regulator of the growth of hematopoietic stem and progenitor cells [Citation8]. In HL, elevated levels strongly predicted shorter free-from-progression survival and overall survival of the patients [Citation10]. TNF-α and its receptors were also elevated in patients with NHL and could represent valuable prognostic markers in those individuals [Citation11,Citation12]. Studies also showed that TNF-α may cooperate with other cytokines, such as IL-6, IL-10, and IL-2 in vivo to increase NHL cell proliferation [Citation13]. In myeloma, TNF-α plays an important role in the proliferation and survival of plasma cells [Citation14] as reduced apoptosis rate is mediated partly by TNF-α [Citation15,Citation16]. A study has found that mean level of TNF-α was significantly higher in AML patients than in the controls [Citation17]. High serum TNF-α level is an adverse prognostic factor for overall and event-free survival in AML patients [Citation7]. However, in our study plasma TNF-α level did not show any statistically significant associations with mortality of any of patient groups.
IL-6 plays a vital role in solid and hematological tumors, as elevated serum levels of IL-6 are often correlated with adverse prognosis, and probably contribute to constitutional manifestations as weight loss, night sweats, fever, and other paraneoplastic symptoms [Citation18,Citation19]. IL-6’s role as a growth and survival factor in multiple myeloma is well established [Citation20]. IL-6 is also a potent osteoclast-activating factor, and contributes to the development of bone lesions. Elevated levels of IL-6 and soluble IL-6 receptors frequently present in the BM, plasma, and serum of myeloma patients are considered as a poor prognostic indicator [Citation9]. High levels of IL-6 are also present in Castleman’s disease, and are associated with severe inflammatory symptoms, increased levels of acute-phase proteins, and hypergammaglobulinemia [Citation21]. In many cases, serum IL-6 or sIL-6R levels are elevated for low- and high-grade non-Hodgkin’s lymphomas, Hodgkin’s disease, and in adult T-cell leukemia/lymphoma [Citation9]. Our study emphasized the aforementioned findings with myeloma and higher level of serum IL-6 was significantly associated with increased mortality in patients with plasma cell dyscrasias; further studies are needed to clarify the potential use of IL-6 as a prognostic marker and its inclusion in myeloma scoring system, and to elaborate the use of IL6 antagonists in myeloma therapeutic strategies.
Elevated D-dimer levels are not specific and may also be observed in many clinical settings, such as cancer, pregnancy, and infectious diseases or following trauma and surgery [Citation5]. In Ay’s study published in 2012, high D-dimer levels were associated with poor overall survival and increased mortality risk in cancer patients including patients with hematological malignancies (p value was 0.001 in lymphoma and 0.65 in myeloma) [Citation5]. Yet, in our study, D-dimer did not show any statistical significance with any of the hematological malignancies concerning mortality.
Fibrinogen exerts well-known roles in hemostasis as a substrate for fibrin clot formation and by binding to platelets to trigger platelet aggregation. Additionally, fibrinogen acts as an acute-phase protein and is elevated during inflammatory processes [Citation22]. In 2016, Berger et al. showed that overall survival in AML was significantly better in the low fibrinogen level (27.3 vs 13.5 months; p = 0.0009) as well as progression-free survival (12.3 vs 7.8 months; p = 0.0076) [Citation23]. However, in our study fibrinogen levels did not show any prognostic impact on hematological malignancies.
Antithrombin is a hepatocyte-produced protein with both intravascular and extravascular coagulation properties, and it is present normally in blood and lymph both in soluble form and bonded to thrombin-forming reversible antithrombin–thrombin complexes [Citation24]. Some studies showed lower concentrations of antithrombin in advanced disease with poorer prognosis [Citation25–27]. In studies including patients with lymphoma, lowered levels of serum antithrombin have been characteristically discovered in active disease. Whereas successful treatment appears to normalize values, but there are still statistically significant differences between groups of lymphoma patients versus controls [Citation28]. Our study supports these findings and lower antithrombin is associated with poorer prognosis in lymphoproliferative malignancies (p value: 0.004*). This can be explained by reduced hepatic synthesis of antithrombin in lymphoma patients, as it was also found in our study that lower serum albumin levels have a negative impact on our patients with lymphoproliferative disorders, in addition to the fact that elevated thrombin production in cancer patients would bind with soluble thrombin, causing its increased consumption. This finding could encourage further studies to elaborate the potential use of antithrombin and albumin as prognostic markers in lymphoproliferative disorders.
PAI-1 is an essential component of the plasminogen–plasmin system and involved in the regulation of many physiologic processes and in the pathogenesis of many disorders including cancer [Citation29,Citation30]. PAI-1 levels can be used as a prognostic marker for thromboembolic complications in patients with cancer, and veno-occlusive disease in the post-bone marrow transplantation setting [Citation29]. Although elevated levels of PAI-1 have been found in leukemic cells, mainly in lysates of myeloid origin [Citation31], but to the best of our knowledge, no studies were conducted to demonstrate the impact of high levels of PAI-1 on the prognosis of hematological malignancies and our study did not demonstrate any relation between serum PAI-1 levels and prognosis of blood cancers.
Higher plasma vWF concentrations are recognized in solid tumors and associated with advanced tumor stage and poorer prognosis [Citation32–34]. A recently published study showed elevated vWF level in 45 patients with leukemia and lymphoma [Citation6]. In 2003, Minnema et al. showed that Higher FVIII:C/VWF-Ag levels are found in patients with active myeloma [Citation35] and in a previously published study of 72 patients with Waldenström’s macroglobulinemia (WM) surprisingly very high VWF:Ag was found in 59% of patients with an association with shorter survival [Citation36]. Also Kastritis et al. showed, in 2016, that high vWF:Ag levels are associated with poorer prognosis in patients with AL amyloidosis, independently of other features of the disease or cardiac biomarkers [Citation37]. In our study, plasma cell dyscrasias patients had poorer prognosis with higher level of vWF (p value: 0.006) as those patients are likely to have more active disease with more microenvironment interactions between plasma cells and endothelial cells and increased bone marrow angiogenesis. We suggest that VWF:Ag may provide additional prognostic information to the present international scoring systems for myeloma and WM (ISS myeloma, ISSWM) which are built as a combination of age and covariates mainly related to tumor burden and adverse cytogenetics.
Selectins are lectin-like adhesion molecules and are involved in lymphocyte extravasation, especially during inflammation and cancer metastasis. Increased soluble P-selectin concentrations are associated with VTE [Citation38]. Concerning hematological malignancies, Muz at al. showed that inhibiting the interaction between myeloma cells and endothelial and stromal cells via P-selectin inhibitors decreased proliferation in myeloma cells [Citation39]. It was found also that soluble L selectin is expressed and released by AML blast cells and soluble E and soluble L selectins and cellular L selectin may be useful prognostic markers in evaluating AML patients at diagnosis [Citation40]. However, P-selectin level did not show any prognostic impact on our patients.
Serum albumin provides a simple estimator of visceral protein function. Malnutrition and inflammation suppress albumin synthesis [Citation41]. Proinflammatory cytokines and growth factors are released as part of the systemic inflammatory response to the tumor, and lower serum albumin concentration may be due to the production of cytokines, such as IL-6, which modulate the production of albumin by hepatocytes [Citation42]. It is well known that serum albumin is an important prognositic marker in myeloma [Citation43]. In our study, lower serum albumin was significantly associated with poorer prognosis in lymphoproliferative neoplasms and plasma cell dyscrasias and this goes in accordance with myeloma international scoring system; however, albumin is not included in any of the current lymphoproliferative disorders prognostic systems.
Limitations of this study included the relative small number of patients, which caused the non-parametric nature of some variables, practical uneasiness of post-mortem autopsy as a tool for the diagnosis of vascular events as a cause of sudden unexplained death; also hereditary thrombophilia screening was not performed for the patients.
In conclusion, some biomarkers of coagulation and inflammation showed statistically significant associations with hematological malignancies concerning plasma cell dyscrasias mortality, vWF with a cut-off level of >3.31 mU/ml (reflecting increased angiogenesis), and IL-6 with a cut-off level of >5.15 pg/ml (with the possibility of using of IL6 antagonists in therapeutic strategies) may provide additional prognostic information to the present international scoring systems for myeloma and WM (ISS myeloma, ISSWM). With lymphoproliferative disorders mortality, antithrombin with a cut-off level of <16.25 mg/dl (due to reduced hepatic synthesis and increased consumption) potentially could be added to the current prognostic scores.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Shereef Elmoamly http://orcid.org/0000-0002-1765-9587
References
- Trousseau A. Lectures on clinical medicine delivered at the Hotel-Dieu, Paris [translated by Cormack JR]. 5th ed. London: New Sydenham Society; 1872. p. 281–295.
- Dvorak HF, Rickles FR. Malignancy and hemostasis. In: VJ Marder, AW Clowes, JN George, et al. editor. Hemostasis and thrombosis, basic principles and clinical practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. p. 851–873.
- Falanga A, Russo L. Epidemiology, risk and outcomes of venous thromboembolism in cancer. Hämostaseologie. 2012;32:115–125. doi: 10.5482/ha-1170
- Rickles FR, Falanga A. Activation of clotting factors in cancer. Cancer Treat Res. 2009;148:31–41. doi: 10.1007/978-0-387-79962-9_3
- Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–1164. doi: 10.3324/haematol.2011.054718
- Mohren M, Jentsch-Ullrich K, Koenigsmann M, et al. High coagulation factor VIII and von Willebrand factor in patients with lymphoma and leukemia. Int J Hematol. 2016;103:189–195. doi: 10.1007/s12185-015-1913-y
- Tsimberidou AM, Estey E, Wen S, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer. 2008;113:1605–1613. doi: 10.1002/cncr.23785
- Tian T, Wang M, Ma D. TNF-α, a good or bad factor in hematological diseases? Stem Cell Investig. 2014;1:12.
- Burger R. Impact of interleukin-6 in hematological malignancies. Transfus Med Hemother. 2013;40:336–343. doi: 10.1159/000354194
- Warzocha K, Bienvenu J, Ribeiro P, et al. Plasma levels of tumour necrosis factor and its soluble receptors correlate with clinical features and outcome of Hodgkin’s disease patients. Br J Cancer. 1998;77:2357–2362. doi: 10.1038/bjc.1998.391
- Salles G, Bienvenu J, Bastion Y, et al. Elevated circulating levels of TNFalpha and its p55 soluble receptor are associated with an adverse prognosis in lymphoma patients. Br J Haematol. 1996;93:352–359. doi: 10.1046/j.1365-2141.1996.5181059.x
- Warzocha K, Salles G, Bienvenu J, et al. Tumor necrosis factor ligand-receptor system can predict treatment outcome in lymphoma patients. J Clin Oncol. 1997;15:499–508. doi: 10.1200/JCO.1997.15.2.499
- Voorzanger N, Touitou R, Garcia E, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–5505.
- Jourdan M, Tarte K, Legouffe E, et al. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw. 1999;10:65–70.
- Jurisić V, Colović M. Correlation of sera TNF-alpha with percentage of bone marrow plasma cells, LDH, beta2- microglobulin, and clinical stage in multiple myeloma. Med Oncol. 2002;19:133–139. doi: 10.1385/MO:19:3:133
- Jöhrer K, Janke K, Krugmann J, et al. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine upregulation of MCP-1. Clin Cancer Res. 2004;10:1901–1910. doi: 10.1158/1078-0432.CCR-1053-03
- Sanchez-Correa B, Bergua JM, Campos C, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–891. doi: 10.1016/j.cyto.2012.12.023
- Mihara M, Hashizume M, Yoshida H, et al. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122:143–159. doi: 10.1042/CS20110340
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999
- Klein B, Zhang XG, Lu ZY, et al. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–872.
- Burger R, Wendler J, Antoni K, et al. Interleukin-6 production in B-cell neoplasias and Castleman's disease: evidence for an additional paracrine loop. Ann Hematol. 1994;69:25–31. doi: 10.1007/BF01757344
- Hoppe B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thromb Haemost. 2014;112:649–658. doi: 10.1160/TH14-01-0085
- Berger MD, Heini AD, Seipel K, et al. Increased fibrinogen levels at diagnosis are associated with adverse outcome in patients with acute myeloid leukemia. Hematol Oncol. 2016. doi: 10.1002/hon.2307
- Hensen A, Loeliger EA. Antithrombin III. Its metabolism and its function in blood coagulation. Thromb Diath Haemorrh. 1963;83(Suppl 1):1–84.
- Dixit A, Kannan M, Mahapatra M, et al. Roles of protein C, protein S, and antithrombin III in acute leukemia. Am J Hematol. 2006;81:171–174. doi: 10.1002/ajh.20546
- Sun W, Ren H, Gao C, et al. Clinical and prognostic significance of coagulation assays in pancreatic cancer patients with absence of venous thromboembolism. Am J Clin Oncol. 2015;38(6):550–556. doi: 10.1097/01.coc.0000436088.69084.22
- Iwako H, Tashiro H, Amano H, et al. Prognostic significance of antithrombin III levels for outcomes in patients with hepatocellular carcinoma after curative hepatectomy. Ann Surg Oncol. 2012;19:2888–2896. doi: 10.1245/s10434-012-2338-y
- Kuusisto ML, Haapasaari KM, Remes AM, et al. Antithrombin III is probably not a suitable biomarker for diagnosis of primary central nervous system lymphoma. Ann Hematol. 2015;94:1167–1174. doi: 10.1007/s00277-015-2334-y
- Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets. 2007;8(9):962–970. doi: 10.2174/138945007781662364
- Stefansson S, McMahon GA, Petitclerc E, et al. Plasminogen activator inhibitor-1 in tumor growth, angiogenesis and vascular remodeling. Curr Pharm Des. 2003;9(19):1545–1564. doi: 10.2174/1381612033454621
- Wada H, Kumeda Y, Ogasawara Z, et al. Plasminogen activators and their inhibitors in leukemic cell homogenates. Am J Hematol. 1993;42:166–170. doi: 10.1002/ajh.2830420205
- Wang WS, Lin JK, Lin TC, et al. Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol. 2005;11:2166–2170. doi: 10.3748/wjg.v11.i14.2166
- Yang X, Sun H, Li Z, et al. Gastric cancer-associated enhancement of von Willebrand factor is regulated by vascular endothelial growth factor and related to disease severity. BMC Cancer. 2015;15:49. doi: 10.1186/s12885-015-1042-2
- Röhsig LM, Damin DC, Stefani SD, et al. von Willebrand factor antigen levels in plasma of patients with malignant breast disease. Braz J Med Biol Res. 2001;34(9):1125–1129. doi: 10.1590/S0100-879X2001000900004
- Minnema MC, Fijnheer R, De Groot PG, et al. Extremely high levels of von Willebrand factor antigen and of procoagulant factor VIII found in multiple myeloma patients are associated with activity status but not with thalidomide treatment. J Thromb Haem. 2003;1:445–449. doi: 10.1046/j.1538-7836.2003.00083.x
- Hivert B, Caron C, Petit S, et al. Clinical and prognostic implications of low or high level von Willebrand factor in patients with Waldenström macroglobulinemia. Blood. 2012;120:3214–3221. doi: 10.1182/blood-2011-11-388256
- Kastritis E, Papassotiriou I, Terpos E, et al. Clinical and prognostic significance of serum levels of von Willebrand factor and ADAMTS-13 antigens in AL amyloidosis. Blood. 2016;128(3):405–409. doi: 10.1182/blood-2016-02-702696
- Ay C, Jungbauer LV, Sailer T, et al. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin Chem. 2007;53:1235–1243. doi: 10.1373/clinchem.2006.085068
- Muz B, Azab F, de la Puente P, et al. Inhibition of P-selectin and PSGL-1 using humanized monoclonal antibodies increases the sensitivity of multiple myeloma cells to bortezomib. Biomed Res Int. 2015;2015:417–586. doi: 10.1155/2015/417586
- Aref S, Salama O, Al-Tonbary Y, et al. L and E selectins in acute myeloid leukemia: expression, clinical relevance and relation to patient outcome. Hematology. 2002;7(2):83–87. doi: 10.1080/10245330290028579
- Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32:S118–S125. doi: 10.1016/S0272-6386(98)70174-X
- Barber MD, Ross JA, Fearon KC. Changes in nutritional, functional, and inflammatory markers in advanced pancreatic cancer. Nutr Cancer. 1999;35:106–110. doi: 10.1207/S15327914NC352_2
- Kim JE, Yoo C, Lee DH, et al. Serum albumin level is a significant prognostic factor reflecting disease severity in symptomatic multiple myeloma. Ann Hematol. 2010;89:391–397. doi: 10.1007/s00277-009-0841-4