ABSTRACT
Objective: Two distinct forms of FMS-like tyrosine kinase 3 (FLT3) mutations, internal tandem duplication (ITD) in the juxtamembrane domain and point mutation within the activation loop of the tyrosine kinase domain (TKD), have been identified in considerable number of patients with AML. This study was aimed to analyze the impacts of these mutations on clinical outcomes, and assess the efficacy of different therapeutic regimens (allo-HSCT, sorafenib, or conventional chemotherapy) for AML patients with FLT3 mutations after the standard induction therapy.
Materials and methods: We analyzed DNA samples from 158 consecutive de novo AML patients (18–60 years, excluding APL) with FLT3 mutations between July 2010 and October 2015.
Results: We found that AML patients with FLT3-TKD mutations have more favorable clinical outcomes than those with FLT3-ITD mutations. We also found that allo-HSCT therapy subgroup achieved longer OS and RFS than non-allo-HSCT therapy subgroup for FLT3-ITD positive patients (p < 0.001, p = 0.071). However, compared with the clinical outcomes in non-primary refractory patients, sorafenib did not show an obvious beneficial effect for the primary refractory patients. Further study on a large scale is still recommended.
Conclusions: FLT3-TKD-mutated AML patients have more favorable clinical outcomes than those with FLT3-ITD mutations. Allo-HSCT therapy subgroup achieved longer OS and RFS than non-allo-HSCT therapy subgroup for FLT3-ITD positive patients. Compared with the clinical outcomes in non-primary refractory patients, sorafenib did not show an obvious beneficial effect for the primary refractory patients.
Introduction
FLT3, also known as human stem cell kinase-1 (STK-1) and fetal liver kinase-2 (FLK-2), belongs to the class III receptor tyrosine kinase (RTK) family [Citation1]. The human FLT3 gene is located on chromosome 13q12 and encompasses 24 exons. It encodes a 993 amino acid protein, which contains an extracellular domain at the N-terminus, a single transmembrane region, an intracellular juxtamembrane domain (JMD), and two kinase domains at the C-terminus [Citation2,Citation3].
FLT3 is primarily expressed in immature hematopoietic cells, particularly in CD34+ hematopoietic stem cell, early progenitor cells, and dendritic cell progenitors [Citation3–5]. It is also found in other tissues such as placenta, gonads, and brain, though its function in these tissues remains unclear [Citation2]. The ligand for FLT3 (FLT3 ligand or FL) is a type I transmembrane protein and expressed in many different human tissues, but its co-expression with FLT3 is limited to the gonads and hematopoietic tissue [Citation6–8]. In normal human bone marrow (BM), FL induces dimerization, activation, and autophosphorylation of the receptor and subsequent activation of several signaling pathways, including STAT5, phosphatidylinositol 3 kinase (PI3K)/AKT, Ras/mitogen-activated protein kinase (MAPK) pathways [Citation9,Citation10].
FLT3 is aberrantly expressed in a wide range of hematopoietic malignancies including AML (70–100%) and ALL, which indicate that FLT3 expression may play an important role in the survival or proliferation of leukemic blasts [Citation11]. Meanwhile, two distinct forms of FLT3 mutations have been identified, internal tandem duplication (ITD) in the JMD and point mutation within the activation loop of the tyrosine kinase domain (TKD). Nakao et al. first reported the presence of ITDs in the JMD domain of FLT3 in AML in 1996 [Citation12]. Later, these ITD mutations were confirmed by many groups. The overall frequency of FLT3-ITD mutations is approximately 20–35% in adult AML and 5–15% in newly diagnosed pediatric AML patients [Citation13–15]. FLT3-ITD mutations have also been detected at lower frequency in myelodysplastic syndrome, but rarely detected in ALL patients [Citation16]. FLT3-TKD mutations were first described in de novo human leukemia in 2001 [Citation17,Citation18]. In large retrospective studies, TKD mutations were present in ∼7% of both adult and pediatric de novo AML [Citation15,Citation18,Citation19]. Interestingly, both FLT3-ITD and FLT3-TKD mutations result in FL-independent constitutive activation of the receptor and downstream pathways [Citation1,Citation12,Citation20,Citation21].
FLT3-ITD mutations are indicated as an independent variable by the majority of retrospective data that confer a poor prognosis in AML [Citation22,Citation23]. In contrast to patients with wild-type FLT3 alleles (FLT3-WT), FLT3-ITD positive patients are associated with poor overall survival (OS) and recurrence free survival (RFS) [Citation15,Citation23,Citation24]. After the standard induction therapy, FLT3 mutated patients are often referred for allogeneic hematopoietic stem cell transplantation (allo-HSCT) and FLT3-specific tyrosine kinase inhibitors (TKIs) treatment (such as sorafenib) [Citation22,Citation25]. However, the efficacy of different therapeutic regimens for FLT3-ITD positive patients after the standard induction therapy is still uncertain, particularly in Chinese patients. Because the occurrence of FLT3-TKD mutations is relatively infrequent, its prognostic relevance remains unknown [Citation22,Citation26–28].
In the present study, we determined the prevalence of both FLT3-ITD and FLT3-TKD mutations in a large group of de novo AML patients, and analyzed the impacts of these mutations on clinical outcomes. We also assessed the efficacy of different therapeutic regimens (allo-HSCT, sorafenib, or conventional chemotherapy) for AML patients with FLT3 mutations after the standard induction therapy.
Materials and methods
Patients and materials
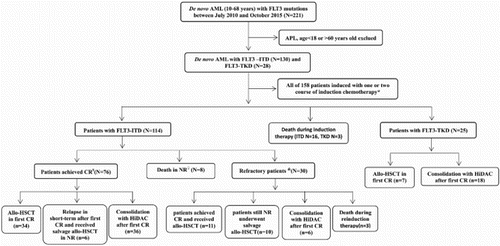
Diagnostic BM samples were analyzed from 158 consecutive de novo AML patients (18–60 years, excluding APL) with FLT3 mutations between July 2010 and October 2015. Studies were approved by the ethics committee of the First Affiliated Hospital of Soochow University (FAHSU) in accordance with the declaration of Helsinki protocol. Of the 158 patients, 130 (82.3%) had FLT3-ITD mutations, 28 (17.7%) had FLT3-TKD mutations.
Patients were first treated with the standard intensive induction therapy consists of anthrycline and cytarabine. Based on FLT3 mutation types, induction results, and economic conditions, patients were then treated with different therapeutic regimens ().
Detection of FLT3 mutations
All samples investigated in this study were obtained at the time of diagnosis. Genomic DNA was extracted from BM samples according to the manufacture’s protocols (QIAGEN AllPrep DNA/RNA mini kit; QIAGEN). As the location of FLT3-ITD is restricted to exons 14 and 15, polymerase chain reaction (PCR) was performed using the method described by Meshinchi et al. [Citation23]. In brief, 100 ng of DNA was amplified in a volume of 50 µl containing 50 mM KCl, 10 mM Tris–HCl, pH 8.3, 1.5 mM MgCl2, 0.001% (wt/vol) gelatin, 200 µM dNTPs, 0.5 µM of each oligonucleotide (FLT3-ITDF: 5′-GCAATTTAGGTATGAAAGCCAGC-3′ and FLT3-ITDR: 5′-CTTTCAGCATTTTGACGGCAACC-3′), and 1 unit Taq DNA polymerase (QIAGEN, Hilden, Germany). The PCR consisted of an initial incubation step at 95°C for 180 seconds, followed by 35 cycles at 95°C for 30 seconds, 57°C for 30 seconds, 72°C for 90 seconds, and a final elongation step at 72°C for 10 minutes. PCR products were analyzed on standard 3% agarose gels. A portion of each PCR products were sequenced using an ABI 3730 automated DNA sequencer (Applied Biosystems, Foster City, CA, U.S.A.). PCR for detection of FLT3-TKD mutations was performed using the same method as above, and the oligonucleotides are: 5′-CCACATCAACGCTATGAGGTAA-3′ (FLT3-TKDF) and 5′-CAGCCCAGTAAAGATAAGAGGC-3′ (FLT3-TKDR).
Statistical analysis
Patient-, disease-related characteristics were compared among the different groups by using the χ2 test for categorical variables and independent sample t-test for continuous variables. The Kaplan–Meier method was used to estimate the univariate probabilities of the OS and RFS. The log-rank test was used to compare the survival curves. All the statistical analyses were performed using SPSS 19 statistical software. p-Values below 0.05 were considered statistically difference.
Results
Patient characteristics at diagnosis
Among 158 de novo AML patients with FLT3 mutations, 130 (82.3%) had FLT3-ITD mutations, 28 (17.7%) had FLT3-TKD mutations. Clinical variables such as sex, age, WBC counts, hemoglobin counts, platelet counts, percentage of BM blasts, karyotype, as well as the presence of cooperating mutations do not differ significantly between FLT3-ITD and FLT3-TKD mutations (). More patients (64.3%) in the FLT3-TKD group achieved a complete remission (CR) after the first course of induction therapy compared to patients of the FLT3-ITD group (46.2%), but this difference is not statistically significant (p = 0.082).
Table 1. Characteristic of patients with AML according to FLT3 mutation type.
Survival comparison of FLT3 mutated AML patients
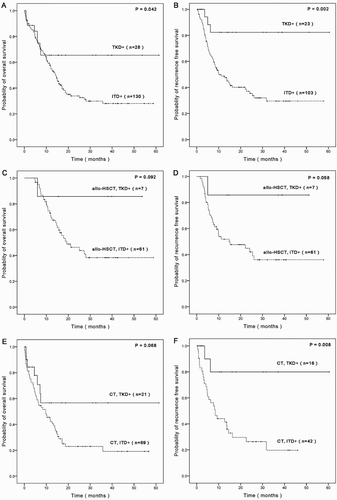
FLT3-TKD positive AML patients had significantly longer OS and RFS than FLT3-ITD positive patients (p = 0.042, p = 0.002) (A,B). The estimated OS rates at 3 years for FLT3-ITD and FLT3-TKD groups were 28.0 and 65.4%, respectively (A). To take a closer view, FLT3-ITD and FLT3-TKD-mutated patients were divided into allo-HSCT subgroups and CT subgroups. Based on the analysis, FLT3-TKD positive AML patients still had longer OS and RFS than FLT3-ITD positive patients, no matter for the allo-HSCT subgroups or for the CT subgroups (C–F).
Figure 1. Survival comparison of FLT3 mutated AML patients. A/B. Comparison of OS and RFS for ITD positive group and TKD positive group. C/D. Comparison of OS and RFS for allo-HSCT, ITD positive subgroup and allo-HSCT, TKD positive subgroup. E/F. Comparison of OS and RFS for CT, ITD positive subgroup and CT, TKD positive subgroup.
Efficacy of allo-HSCT in FLT3 mutated AML patients
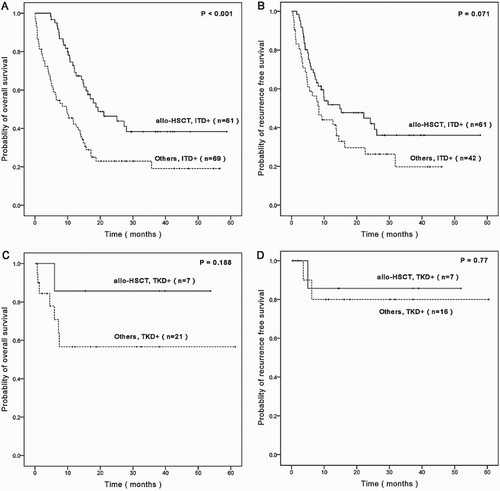
To analyze the efficacy of allo-HSCT in patients with FLT3-ITD or FLT3-TKD mutations, patients were divided into allo-HSCT subgroup and other subgroup. For FLT3-ITD mutated patients, allo-HSCT subgroup achieved significantly longer OS than other patients (p < 0.001), the estimated OS rate at 3 years for allo-HSCT subgroup and other subgroup were 38.3 and 19.1%, respectively (A). Although allo-HSCT subgroup also achieved longer RFS than other patients (36.1 versus 19.7%), the difference is not statistically significant (p = 0.071) (B). For FLT3-TKD positive patients, there was no significantly difference in OS and RFS between allo-HSCT subgroup and other subgroup (p = 0.188, p = 0.77) (C,D), although the estimated OS rates at 3 years for allo-HSCT subgroup and other subgroup were 85.7 and 56.6%, respectively.
Efficacy of sorafenib in FLT3-ITD mutated refractory AML patients
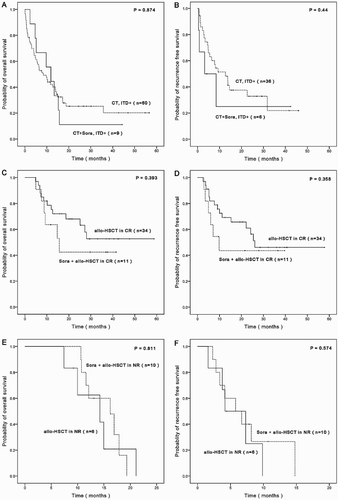
To determine the efficacy of sorafenib in refractory patients with FLT3-ITD mutations, patients were divided into sorafenib group (refractory patients) (n = 30) and other group (n = 100), and then were subdivided into CT subgroup (n = 60), CT + Sora subgroup (n = 9), allo-HSCT in CR subgroup (n = 34), Sora + allo-HSCT in CR subgroup (n = 11), allo-HSCT in NR subgroup (n = 6), and Sora + allo-HSCT in NR subgroup (n = 10). Based on the statistical data analysis, Compared with the clinical outcomes in non-primary refractory patients, sorafenib did not show an obvious beneficial effect for the primary refractory patients, whether for the CT subgroup or for the allo-HSCT subgroup ().
Figure 3. Efficacy of sorafenib in FLT3-ITD mutated refractory AML patients. A/B. Comparison of OS and RFS for CT subgroup and CT + Sora subgroup. C/D. Comparison of OS and RFS for allo-HSCT in CR subgroup and allo-HSCT + Sora in CR subgroup. E/F. Comparison of OS and RFS for allo-HSCT in NR subgroup and allo-HSCT + Sora in NR subgroup.
Discussion
Despite numerous reports on the clinical significance of FLT3 mutations, survival analysis of AML patients between FLT3-ITD and FLT3-TKD positive groups is still lacking, especially in Chinese populations. The therapeutic effect of different therapies was also not completely elucidated. In the present study, we show that AML patients (APL excluded) with FLT3-TKD mutations have more favorable clinical outcomes than those with FLT3-ITD mutations. We also show that allo-HSCT therapy subgroup achieved longer OS and RFS than non-allo-HSCT therapy subgroup for FLT3-ITD positive patients (p < 0.001, p = 0.071). However, sorafenib did not show obvious beneficial effect for the refractory patients.
Because the occurrence of FLT3-TKD mutations is relatively infrequent, its prognostic relevance remains to be elucidated. There were 28 FLT3-TKD positive de novo AML patients chosen in this study. From the results, FLT3-TKD positive patients have more favorable clinical outcomes than those with FLT3-ITD mutations, when compared with either the whole group, or divided subgroups by different therapeutic regimens. These results confirmed the findings of previous studies with meta-analysis methods [Citation29,Citation30]. Since the proteins coded by both FLT3-ITD and FLT3-TKD mutations exhibit FL-independent FLT3 dimerization and constitutive activation through autophosphorylation, what makes the FLT3-ITD mutations subjected to worse clinical outcomes is an important issue. In future studies, we would test the kinase activities of different FLT3-ITD and FLT3-TKD proteins and determine the relationship between activity and clinical outcomes.
In the recent years, allo-HSCT is often recommended for patients with FLT3 mutated AML [Citation31], especially in FLT3-ITD positive patients. However, the efficacy of allo-HSCT for FLT3-ITD positive AML is still controversial [Citation32–35]. In our study, the estimated OS rates at 3 years for allo-HSCT subgroup was significantly higher than non-allo-HSCT subgroup (p < 0.001), and allo-HSCT subgroup achieved longer RFS than non-allo-HSCT subgroup (p = 0.071). Surprisingly, we did not see significantly difference of OS and RFS between allo-HSCT subgroup (n = 7) and other subgroup (n = 21) in FLT3-TKD positive patients (p = 0.188, p = 0.77), although the estimated OS rates at 3 years for allo-HSCT subgroup and CT subgroup were 85.7 and 56.6%, respectively. Further studies are needed to confirm or disprove this conclusion by larger samples. Our results suggest that allo-HSCT improves outcomes especially in patients with FLT3-ITD, but not FLT3-TKD.
Sorafenib is considered effective in AML patients carrying FLT3-ITD mutations [Citation36]. Unfortunately, from our results, compared with the clinical outcomes in non-primary refractory patients, sorafenib did not show an obvious beneficial effect on OS and RFS for the primary refractory patients with FLT3-ITD mutations (). It should be noted that this study has only examined if FLT3-ITD mutation is positive or not, we did not further detect its mutation level, location, size and other variables which may impact the clinical outcomes and conclusions.
Conclusions
In conclusion, this study shows that AML patients (APL excluded) with FLT3-TKD mutations have more favorable clinical outcomes than those with FLT3-ITD mutations. It also shows that allo-HSCT therapy subgroup achieved longer OS and RFS than non-allo-HSCT therapy subgroup for FLT3-ITD positive patients (p < 0.001, p = 0.071). However, compared with the clinical outcomes in non-primary refractory patients, sorafenib did not show an obvious beneficial effect for the primary refractory patients. Further study on a large scale is still recommended.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492
- Rosnet O, Schiff C, Pebusque MJ, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82(4):1110–1119.
- Matthews W, Jordan CT, Wiegand GW, et al. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-V
- Kuczynski WI, Ratajczak MZ. STK-1 receptor gene is expressed in various human non-hematopoietic tumor cell lines--RT-PCR directed analysis of STK-1 mRNA expression. Preliminary report. Acta Haematol Pol. 1994;25(1):43–46.
- McKenna HJ. Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr Opin Hematol. 2001;8(3):149–154. doi: 10.1097/00062752-200105000-00004
- Lyman SD, James L, Johnson L, et al. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83(10):2795–2801.
- Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-K
- Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368(6472):643–648. doi: 10.1038/368643a0
- Lyman SD. Biology of flt3 ligand and receptor. Int J Hematol. 1995;62(2):63–73. doi: 10.1016/0925-5710(95)00389-A
- Zhang S, Fukuda S, Lee Y, et al. Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J Exp Med. 2000;192(5):719–728. doi: 10.1084/jem.192.5.719
- Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10(4):588–599.
- Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918.
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111(1):190–195. doi: 10.1046/j.1365-2141.2000.02317.x
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.V98.6.1752
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.V99.12.4326
- Horiike S, Yokota S, Nakao M, et al. Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia. 1997;11(9):1442–1446. doi: 10.1038/sj.leu.2400770
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113(4):983–988. doi: 10.1046/j.1365-2141.2001.02850.x
- Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–2439. doi: 10.1182/blood.V97.8.2434
- Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108(12):3654–3661. doi: 10.1182/blood-2006-03-009233
- Kiyoi H, Ohno R, Ueda R, et al. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21(16):2555–2563. doi: 10.1038/sj.onc.1205332
- Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 d835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2007;111(3):1552–1559. doi: 10.1182/blood-2007-08-107946
- Annesley CE, Brown P. The biology and targeting of FLT3 in pediatric leukemia. Front Oncol. 2014;4:1663. doi: 10.3389/fonc.2014.00263
- Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97(1):89–94. doi: 10.1182/blood.V97.1.89
- Kim Y, Lee GD, Park J, et al. Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 2015;5:e336. doi: 10.1038/bcj.2015.61
- Pratz KW, Levis M. How I treat FLT3-mutated AML. Blood. 2017;129(5):565–571. doi: 10.1182/blood-2016-09-693648
- Wang L, Lin D, Zhang X, et al. Analysis of FLT3 internal tandem duplication and D835 mutations in Chinese acute leukemia patients. Leuk Res. 2005;29(12):1393–1398. doi: 10.1016/j.leukres.2005.05.013
- Andersson A, Johansson B, Lassen C, et al. Clinical impact of internal tandem duplications and activating point mutations in FLT3 in acute myeloid leukemia in elderly patients. Eur J Haematol. 2004;72(5):307–313. doi: 10.1111/j.1600-0609.2004.00225.x
- Moreno I, Martin G, Bolufer P, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88(1):19–24.
- Li W, Zhang L, Huang L, et al. Meta-analysis for the potential application of FLT3-TKD mutations as prognostic indicator in non-promyelocytic AML. Leuk Res. 2012;36(2):186–191. doi: 10.1016/j.leukres.2011.08.014
- Yanada M, Matsuo K, Suzuki T, et al. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005;19(8):1345–1349. doi: 10.1038/sj.leu.2403838
- Schiller GJ. Evolving treatment strategies in patients with high-risk acute myeloid leukemia. Leuk Lymphoma. 2014;55(11):2438–2448. doi: 10.3109/10428194.2014.881479
- Lin PH, Lin CC, Yang HI, et al. Prognostic impact of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia patients with internal tandem duplication of FLT3. Leuk Res. 2013;37(3):287–292. doi: 10.1016/j.leukres.2012.10.005
- DeZern AE, Sung A, Kim S, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17(9):1404–1409. doi: 10.1016/j.bbmt.2011.02.003
- Schiller GJ, Tuttle P, Desai P. Allogeneic hematopoietic stem cell transplantation in FLT3-ITD-positive acute myelogenous leukemia: The role for FLT3 tyrosine kinase inhibitors post-transplantation. Biol Blood Marrow Transplant. 2016;22(6):982–990. doi: 10.1016/j.bbmt.2016.01.013
- Gale RE, Hills R, Kottaridis PD, et al. No evidence that FLT3 status should be considered as an indicator for transplantation in acute myeloid leukemia (AML): an analysis of 1135 patients, excluding acute promyelocytic leukemia, from the UK MRC AML10 and 12 trials. Blood. 2005;106(10):3658–3665. doi: 10.1182/blood-2005-03-1323
- Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–198. doi: 10.1093/jnci/djm328