ABSTRACT
Background: Delayed immune reconstitution is an important risk factor for increased susceptibility to fungal pathogens. However, little is known about the association between the recovery of CD4+T cell subsets and invasion fungal infections (IFIs) in patients after allogeneic hematopoietic stem cell transplantation (allo-HSCT). The aim of the study was to analyze the immune reconstitution characteristics of CD4+T cell subsets and their association with the incidence of IFIs over the first 3 months after allo-HSCT.
Methods: Fifty-three patients were included, 13 with IFIs. We assessed CD4+T cell subsets and the mRNA expression of specific transcription factors T-bet, GATA3, RORγt, and Foxp3 in peripheral blood mononuclear cells over three time points. The serum levels of IFN-γ, IL-6, IL-10, and TGF-β were detected using the enzyme-linked immunosorbent assay.
Results: CD4+T cell subsets increased progressively in non-IFI patients after allo-HSCT. In IFI patients, Th17 cell counts were significantly decreased compared to non-IFI patients at 3 months after allo-HSCT. IFI patients showed the lower ratios of RORγt/GATA3 and RORγt/Foxp3 compared with non-IFI patients. In addition, we observed increased levels of IFN-γ and IL-10 in IFI patients after allo-HSCT. In the multivariate analysis, the occurrence of IFIs was independently associated with the incidence of IFIs. Finally, we observed a lower CD4:CD8 ratio in IFI patients and its association with Th17 cells.
Conclusions: These findings supported that Th17 cells may be involved in the immune pathology of IFIs after allo-HSCT.
Introduction
Although the systemic antifungal prophylaxis was routinely administrated, invasion fungal infections (IFIs) remain an important cause of infectious morbidity and mortality of allogenic hematopoietic stem cell transplant recipients [Citation1,Citation2]. The immune reconstitution after allogeneic hematopoietic stem cell transplantation (allo-HSCT) plays a critical role in the prevention of disease recurrence and decrease of mortality, and delayed immune reconstitution is a major risk factor for increased susceptibility to fungal pathogens [Citation3,Citation4]. The early recovery of innate immunity such as myeloid series and natural killer cells occurs rapidly within 100 days after allo-HSCT [Citation5]. The reconstitution of adaptive immunity including B-lymphocytes and T-lymphocytes especially for CD4+T cells remains abnormal for many months [Citation6].
Naïve CD4+T cells differentiate into numerous T cell subsets, such as Th1, Th2, Th17, and regulatory T (Treg) cells, a process regulated by specific transcription factors [Citation7]. Among them, T-bet is a critical regulator of Th1 cell differentiation and Th1 cytokine production [Citation8]. GATA3 is a GATA family transcription factor that controls the differentiation of naïve CD4+T cells into Th2 cells [Citation9]. RORγt is required for the differentiation of Th17 cells and expression of IL-17 [Citation10]. The transcription factor Foxp3 is essential for the development of Treg cells [Citation11]. In general, Th1 and Th17 cells are considered to confer protective immunity against fungal infections, whereas Th2 and Treg cells are deleterious [Citation12–14]. However, there has been a little explicit study about their associations with IFIs in patients after allo-HSCT.
In the study, we observed the immune reconstitution characteristics of CD4+T cell subsets in non-IFI and IFI patients during the first 3 months after allo-HSCT. The mRNA expression of specific transcription factors that control naïve CD4+T cell development was determined. We also analyzed the correlation of CD4+T cell subsets with IFIs in patients after allo-HSCT.
Materials and methods
Patients and samples
Fifty-three patients with advanced hematological malignancies who had undergone allo-HSCT were studied. All patients received treatment at Qingdao Central Hospital between July 2013 and March 2015. Clinical parameters of patients were described in . The study was approved by the Ethic Review Board of Qingdao Central Hospital. All patients gave written informed consent. Peripheral blood mononuclear cells (PBMCs) from patients were isolated using Ficoll-Hypaque gradient from peripheral blood (PB) samples that were collected at 1, 2, and 3 months (M1, M2, and M3) after allo-HSCT.
Table 1 Patient’s characteristics.
Flow cytometry
The enumeration of Th1, Th2, and Th17 cells was measured as described previously [Citation15]. Briefly, the cells were stimulated with phorbol myristate acetate (PMA) and ionomycin (all from Alexis Biochemicals, San Diego, CA, U.S.A.) at 37°C, 5% CO2 for 4 hours. After incubation, the cells were stained with CD3 and CD8 (all from eBioscience, San Diego, CA, U.S.A.). Then cells were fixated, permeabilized, and stained for IFN-γ, IL-4, and IL-17 (all from eBioscience, San Diego, CA, U.S.A.). Isotype controls were used to enable correct compensation and confirm antibody specificity. Th1, Th2, and Th17 cells were identified as CD3+CD8−IFN-γ+, CD3+ CD8−IL-4+, and CD3+ CD8−IL-17+, respectively.
CD4+CD25+Foxp3+T cells were evaluated using the Human Regulatory T-cell Staining Kit (eBioscience, San Diego, CA, U.S.A.) according to the manufacturer’s protocol. PBMCs were incubated with a cocktail of anti-CD4-FITC antibody and anti-CD25-APC antibody for 30 minutes in the dark at 4°C to stain the surface. Then cells were fixated, permeabilized, and stained for Foxp3. Cells were washed twice and resuspended in 300 μl PBS. Samples were run through a FACScan flow cytometer.
Absolute cell levels were calculated using the lymphocyte count from the PB sample and the percentages of the different T cell subpopulations measured in the PBMCs.
Quantitative RT-PCR
Total RNA was extracted from PBMCs using TRIzol Reagent (Invitrogen, Carlsbad, CA, U.S.A.), and the cDNA was synthesized by reverse transcription. Quantitative RT-PCR was performed in duplicate on a LightCycler 2.0 Instrument (Roche Diagnostic, Mannheim, Germany) and analyzed using the comparative 2−ΔCT method as previously described [Citation16]. GAPDH was used as an internal control. The primers for T-bet, GATA3, RORγt, Foxp3, and GAPDH are listed in Supplementary Table 1.
Enzyme-linked immunosorbent assay
The sera from all patients were measured using the Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, U.S.A.) according to the manufacturer’s instructions. The optical density of each sample was determined using a microplate reader at 450 nm, and the mean concentrations of IFN-γ, IL-10, TGF-β, and IL-6 were calculated. All samples were measured in duplicate.
Statistical analysis
The data are expressed as the mean ± S.D. based on triplet experiments. The data were analyzed using the analysis of variance and Student’s t-test (two tailed). The Spearman’s test was used for the correlation analysis. Factors associated with IFIs with a p value lower than 0.05 were further tested in the multivariate analysis by the Cox model. p-Values <0.05 were considered statistically significant.
Results
Patients’ characteristics
A total of 38 males and 15 females, with a median age of 40.5 years, were included in the final analysis. The most diagnoses were acute myelogeous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogeous leukemia (CML), and myelodysplastic syndrome (MDS). Forty-one patients had undergone sibling allo-HSCT, 12 patients were treated with URD-HSCT. Thirteen patients developed IFIs early after allo-HSCT. IFIs were Aspergillus (n = 8), Candida albicans (n = 3) and Candida non-Albicans (n = 2). The clinical syndrome was pneumonia in nine cases, colitis in two cases, and sepsis in two cases. No significant differences were observed in terms of age, sex, diagnosis, conditioning regimen, donors, and acute GVHD in non-IFI and IFI patients ().
Decreased Th17 cells in IFI patients
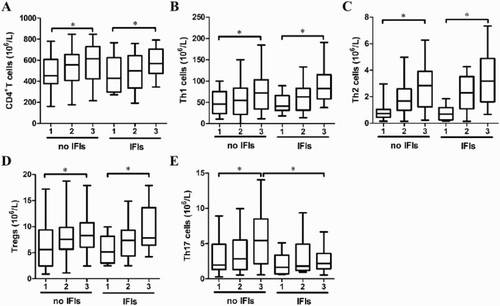
We compared in more details the evolution of different CD4+T cell subsets at early stages after transplantation. The numbers of CD4+T, Th1, Th2, Treg, and Th17 cells increased progressively in non-IFI patients as time went on. Each index at M3 was significantly higher than M1 ((A–E)). As shown in (E), Th17 cell number was significantly higher in non-IFI patients than in IFI patients at M3 after allo-HSCT. There were no significant differences in the numbers of CD4+T, Th1, Th2, and Treg cells between non-IFI and IFI patients ((A–D)).
T-bet, GATA3, RORγt, and Foxp3 mRNA expression in patients with and without IFIs
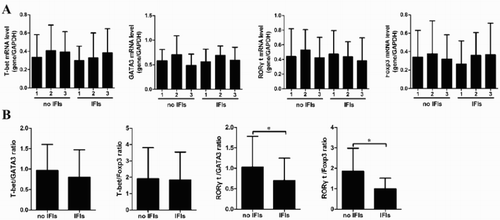
The differentiations of naïve CD4+T cells into Th1, Th2, Treg, and Th17 cells were regulated by specific transcription factors T-bet, GATA3, RORγt, and Foxp3, respectively [Citation17–20]. As shown in (A), no differences were observed in the mRNA expression of T-bet, GATA3, RORγt, and Foxp3 at different time points after transplantation. In addition, we demonstrated no differences in the ratios of T-bet/GATA3 and T-bet/Foxp3 between non-IFI and IFI patients. Interestingly, we found that the ratios of RORγt/GATA3 and RORγt/Foxp3 were decreased in IFI patients at M3 after allo-HSCT compared with non-IFI patients ((B)).
Alteration of serum cytokine levels after transplantation
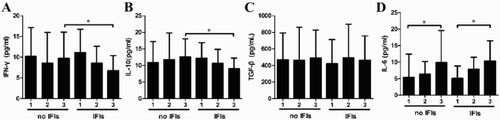
The levels of IFN-γ, IL-10, and TGF-β remained stable in both patient groups at early stages after allo-HSCT, but the level of IL-6 increased gradually ((A–D)). Compared with non-IFI patients, IFN-γ and IL-10 levels were reduced in IFI patients at M3 after allo-HSCT ((A,B)).
Decreased levels of Th17 cells are associated with a higher IFI incidence
A multivariate analysis was adopted to explore the importance of Th17 cell number in the occurrence of IFIs compared with other immunological parameters. Significant factors in the univariate analysis, including RORγt/GATA3, RORγt/Foxp3, IL-10, and IFN-γ were taken into the multivariate analysis, along with Th17 cell number. The results suggested that Th17 cell number remained independently associated with the incidence of IFIs ().
Table 2. Multi-variable analysis of IFIs.
Th17 cells are positively correlated with the CD4:CD8 ratio in IFI patients
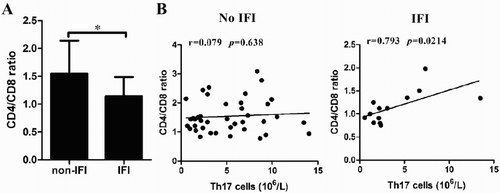
The CD4:CD8 ratio is an important index to evaluate immune status [Citation21,Citation22]. As shown in (A), the CD4:CD8 ratio was decreased in IFI patients compared to non-IFI patients at M3 after allo-HSCT. We determined the correlation of Th17 cells with the CD4:CD8 ratio. As shown in (B), Th17 cells were positively correlated with the CD4:CD8 ratio in IFI patients ().
Discussion
Although it has been reported that delayed immune reconstitution is an important risk factor for increased susceptibility to fungal pathogens, little is known about the association between the recovery of CD4+T cell subsets and IFIs in patients after allo-HSCT. In the study, we investigated CD4+T cell reconstitution and demonstrated that Th17 cells were independently correlated with IFIs at early stages after allo-HSCT.
Th1 and Th17 pathways have recently been shown to play a leading role in anti-fungal activities [Citation23]. Our results showed that the numbers of CD4+T cell subsets increased gradually in the PB of non-IFI patients at early stages after allo-HSCT. No significant differences in the numbers of CD4+T, Th1, Th2, and Treg cells were observed between non-IFI and IFI patients. Interestingly, a significantly decreased Th17 cells was observed in IFI patients at M3 after allo-HSCT compared with non-IFI patients. In addition, Th17 cells were shown to be independently correlated with the occurrence of IFIs by the multivariate analysis. These results suggested that the reduction in Th17 cells might be susceptible to IFIs after allo-HSCT.
Next, we analyzed the expression of T-bet, GATA3, RORγt, and Foxp3, critical transcription factors which control T helper cell differentiation. No significant changes were observed within 3 months after allo-HSCT. We also found that there were no significant differences in the mRNA expression of T-bet, GATA3, RORγt and Foxp3 between non-IFI and IFI patients. The critical transcription factors induced in each lineage are often involved in cross-regulation [Citation24]. We found no significant differences in the ratios of T-bet/GATA3 and T-bet/Foxp3 between non-IFI and IFI patients, whereas the ratios of RORγt/GATA3 and RORγt/Foxp3 were significantly decreased in IFI patients. These results suggested an imbalance of T helper cell differentiation in IFI patients at early stages after allo-HSCT.
Th1-type cells that secret large amount of IFN-γ appear to be critical for promoting fungal clearance, whereas Th2-type cells, which produce IL-4 and IL-6 dampen protective Th1 cell response [Citation25,Citation26]. Treg cells secret TGF-β and IL-10, which limit the protective immune responses [Citation27]. In the study, we found that there were no significant differences in the levels of IFN-γ, IL-10, and TGF-β at different time points after allo-HSCT, whereas IL-6 was elevated significantly. We also determined the cytokines IL-4 and IL-17 and found little amount in the PB at early stages after allo-HSCT (data not shown). The dynamic changes of these cytokines were inconsistent with the recovery of CD4+T helper cell subsets, indicating that these cytokines were independent on CD4+T cell subsets. In addition, we found that IL-10 and IFN-γ levels were significantly higher in IFI patients at M3 after allo-HSCT compared with non-IFI patients. These cytokines are produced not only by CD4+T cell subsets, but also by innate immune cells such as monocytes and granulocytes. Owing to more rapid recovery of innate immunity after allo-HSCT, these cytokines might be related to the activation of innate immune cells.
The CD4/CD8 ratio is associated with markers of immunoactivation, immunosenescence, and T-cell subsets [Citation28]. A low CD4:CD8 ratio may be a sign of immune dysfunction [Citation29]. In the present study, we found that the CD4:CD8 ratio in IFI patients was lower than non-IFI patients at M3 after allo-HSCT. Interestingly, Th17 was positively correlated with the CD8:CD4 ratio in IFI patients. The results in non-IFI patients were dissimilar. The mechanism underlying the correlation between Th17 cells and the CD8:CD4 ratio in IFI patients should be clarified in future studies.
In conclusion, these results suggest that Th17 cells might be a protective factor for anti-fungal infections in patients after allo-HSCT. Abnormal Th17 cell differentiation in IFI patients may be caused by immune dysfunction.
Supplementary_material.docx
Download MS Word (12.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139(4):519–531. doi: 10.1111/j.1365-2141.2007.06812.x
- Salit RB, Shea YR, Gea-Banacloche J, et al. Death by edible mushroom: first report of Volvariella volvacea as an etiologic agent of invasive disease in a patient following double umbilical cord blood transplantation. J Clin Microbiol. 2010;48(11):4329–4332. doi: 10.1128/JCM.01222-10
- Mehta RS, Rezvani K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence. 2016;7(8):901–916. doi: 10.1080/21505594.2016.1208866
- Beltrame M P, Malvezzi M, Bonfim C, et al. Immune reconstitution in patients with Fanconi anemia after allogeneic bone marrow transplantation. Cytotherapy. 2014;16(7):976–989. doi: 10.1016/j.jcyt.2014.02.015
- Peggs KS, Mackinnon S. Immune reconstitution following haematopoietic stem cell transplantation. Br J Haematol. 2004;124(4):407–420. doi: 10.1046/j.1365-2141.2003.04767.x
- Puissant-Lubrano B, Huynh A, Attal M, et al. Evolution of peripheral blood T lymphocyte subsets after allogenic or autologous hematopoietic stem cell transplantation. Immunobiology. 2014;219(8):611–618. doi: 10.1016/j.imbio.2014.03.012
- Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol. 2015;34:130–136. doi: 10.1016/j.coi.2015.03.007
- Szabo SJ, Sullivan BM, Peng SL, et al. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942
- Murphy KM, Reiner SL. Decision making in the immune system: the lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–944. doi: 10.1038/nri954
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490
- Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–288. doi: 10.1038/nri2939
- Romani L, Puccetti P. Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006;14(4):183–189. doi: 10.1016/j.tim.2006.02.003
- Ahlgren KM, Moretti S, Lundgren BA, et al. Increased IL-17A secretion in response to Candida albicans in autoimmune polyendocrine syndrome type 1 and its animal model. Eur J Immunol. 2011;41(1):235–245. doi: 10.1002/eji.200939883
- Wang L, Zhao P, Song L, et al. Correlation of Tc17 cells at early stages after allogeneic hematopoietic stem cell transplantation with acute graft-versus-host disease. Int Immunopharmacol. 2016;41:122–126. doi: 10.1016/j.intimp.2016.11.003
- Zhao P, Gao D, Wang Q, et al. Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4. Cell Mol Immunol. 2015;12(6):692–699. doi: 10.1038/cmi.2014.108
- Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/S0092-8674(00)80702-3
- Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5(11):1157–1165. doi: 10.1038/ni1128
- Han L, Yang J, Wang X, et al. The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor gammat (RORgammat) in Th17 cells. J Biol Chem. 2014;289(37):25546–25555. doi: 10.1074/jbc.M114.565291
- Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019
- Okamoto T, Okada M, Mori A, et al. Correlation between immunological abnormalities and prognosis in myelodysplastic syndrome patients. Int J Hematol. 1997;66(3):345–351. doi: 10.1016/S0925-5710(97)00042-X
- Geiselhart LA, Humphries CA, Gregorio TA, et al. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166(5):3019–3027. doi: 10.4049/jimmunol.166.5.3019
- Garcia-Vidal C, Viasus D, Carratala J. Pathogenesis of invasive fungal infections. Curr Opin Infect Dis. 2013;26(3):270–276. doi: 10.1097/QCO.0b013e32835fb920
- Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238(1):247–262. doi: 10.1111/j.1600-065X.2010.00951.x
- Zhang Y, Wang F, Tompkins KC, et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175(6):2489–2500. doi: 10.2353/ajpath.2009.090530
- Muller U, Stenzel W, Kohler G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179(8):5367–5377. doi: 10.4049/jimmunol.179.8.5367
- Ferreira MC, de Oliveira RT, da Silva RM, et al. Involvement of regulatory T cells in the immunosuppression characteristic of patients with paracoccidioidomycosis. Infect Immun. 2010;78(10):4392–4401. doi: 10.1128/IAI.00487-10
- Sainz T, Serrano-Villar S, Diaz L, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. 2013;27(9):1513–1516. doi: 10.1097/QAD.0b013e32835faa72
- Leung V, Gillis J, Raboud J, et al. Predictors of CD4:CD8 ratio normalization and its effect on health outcomes in the era of combination antiretroviral therapy. PLoS ONE. 2013;8(10):e77665. doi: 10.1371/journal.pone.0077665