ABSTRACT
Background and objective: Sex chromosome loss (SCL) can occur in older men as a physiological phenomenon or as an acquired abnormality in leukemia. Loss of chromosome Y and loss of chromosome X are acquired disorders that are mainly observed in patients over 80 years as well as in myeloid and lymphoid malignancies. In this review, we examine the cytogenetic and molecular changes of sex chromosomes in leukemia.
Methods: Relevant English language literature were searched and retrieved from PubMed search engine (1990–2016). The following keywords were used: ‘Sex chromosomes’, ‘Leukemia’ and ‘Cytogenetics’.
Results: The loss of tumor suppressor genes along with these chromosomal abnormalities in the majority of malignant cells in bone marrow (BM) has raised the question whether this is an age-related phenomenon or has occurred as a result of clonal abnormality. On the other hand, the presence of these chromosomal abnormalities in a number of genetic diseases associated with leukemia leads to progression of malignancy, and their role in peripheral blood stem cell transplantation confirm the finding that these chromosomal abnormalities can play an important role in clonal abnormality.
Conclusion: The presence of these abnormalities can cause genetic instability in BM and result in the development of a malignant clone and progression of the disease. In addition, the evaluation of SCL together with the genes involved in these chromosomes can contribute to predict the disease prognosis as well as monitoring of malignancy.
KEYWORDS:
Highlights
We review the cytogenetic and molecular changes of sex chromosomes in leukemia.
We discuss sex chromosome changes as an age-related or clonal expansion phenomenon in leukemia.
We introduce the cytogenetic and molecular genetics of sex chromosome in the diagnosis and prognosis of leukemia.
Introduction
Loss of sex chromosomes (SCL) applies to the loss of chromosome X (LOX) in women or loss of chromosome Y (LOY) in men, which usually occurs in older people. However, the detection of LOX or LOY in leukemic patients in some cases is indicative of an abnormal clonal karyotype, although raising SCL as a biomarker for prognosis in leukemia is still in its infancy [Citation1]. Association of SCL with other chromosomal abnormalities in leukemia is addressed as an additional chromosomal abnormality (ACA). These disorders may be observed in all body cells of patients or may be acquired, and are known as clonal evolution (CE) [Citation2]. SCL is, of course, seen in healthy subjects and is increased in older ages. LOY has also been reported as a physiological phenomenon in the BM cells of elderly men with no history of hematologic malignancies as well as in several myeloid and lymphoid disorders as an acquired abnormality ( and ) [Citation3]. For example, it has been reported in varying degrees (5.1–16.4%) in myeloid neoplasms such as acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL) and myelodysplastic syndromes (MDS) [Citation4]. Studies have shown that the incidence of myeloid neoplasms, such as AML, CML and MDS, is associated with the old age of patients. On the other hand, LOY is a relatively common disorder, and studies indicate a correlation between its incidence and increasing age range of patients, which mainly affects men over 80 years of age. Therefore, the question of whether LOY is an age-related acquired disorder is further raised [Citation4,Citation5]. Increased use of cytogenetic techniques for the diagnosis and prognosis of leukemia, determination of karyotype in patients and associated clinical outcomes indicates the increasing diagnostic and prognostic value of such techniques in SCL detection. Since the first paper concerning LOY was published in 1970, the clinical significance and relationship between LOY and blood diseases, particularly AML and MDS, has remained unclear [Citation5]. In tumor biology, the loss of specific genetic materials is associated with the loss of some tumor-specific suppressor genes, although it is not clear which part of the Y chromosome harbors these genes () [Citation6]. According to the studies, there is no association between the extent of LOY and tumor development and invasion. On the other hand, the relationship between abnormalities of the Y chromosome in Klinefelter syndrome (KS) and CML and Turner syndrome with AML demonstrates their role in the initiation and formation of a malignant clone [Citation7,Citation8]. The coincidence of LOY and relapse also addresses the role of genes in these sex chromosomes in patients following peripheral blood stem cell transplantation (PBSCT). The changes in the X chromosome have also been reported in patients with malignancies such as MDS [Citation9,Citation10]. Similar to LOY, the incidence of LOX in women is increased with age and thus may not be considered as a marker for malignancy. In contrast, a high rate of LOY has been suggested to be indicative of a clone of malignant cells [Citation11]. The role and mechanism of action of LOY in leukemia is unclear. The pathogenic role of the limited number of genes identified on the Y chromosome has not been indicated in the development of malignancies. In addition, the genes involved in the X chromosome rearrangements are little known to date and their role in the pathogenesis of leukemia has not been recognized [Citation8,Citation12]. Given the challenging importance of the role of LOY and LOX in the process of malignancies, their association with age in BM cells as well as the likely role of genes on these chromosomes in leukemia, in this review, we examine the cytogenetic and molecular aspects of changes in sex chromosomes during leukemia.
Figure 1. Age-related SCL in normal BM cells and the leukemogenesis. In normal cells, with the effect of aging in BM, the cells gradually experience age-related SCL. So, two types of clones as normal cells and SCL ones are created. With the effect of a mutation, ACA, loss of tumor suppressor genes and oncogenes, the pre-leukemic state is created, which will ultimately lead to the clonal expansion and leukemia in which SCL can be seen in all the leukemic cells in BM. In this case, depending on the loss of genes involved in sexual chromosomes, ACA, and other factors, benign or malignant leukemia can be created. If leukemia is benign, the cells return to their normal karyotype and if it is malignant, the cells will continue to proliferate. Abbreviation: BM: bone marrow; SCL: sex chromosome loss; ACA: additional chromosome abnormality; LOY: loss of chromosome Y; LOX: loss of chromosome X.
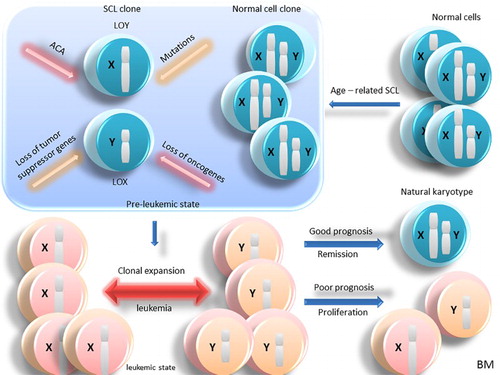
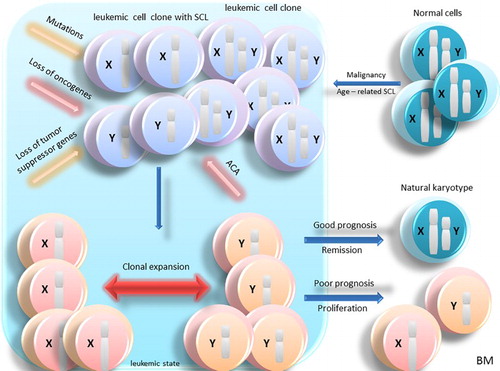
Figure 2. Age-related SCL in leukemic BM cells. In normal cells, age-related SCL due to the effect of aging in BM creates two types of clones: leukemic cells and leukemic cells with SCL. With the effect of mutation, ACA, loss of tumor suppressor genes and oncogenes, clonal expansion and leukemia is developed and SCL can be seen in all the leukemic cells in BM. In this case, depending on the loss of genes involved in sexual chromosomes, ACA and other factors, benign or malignant leukemia can be developed. If leukemia is benign, the cells return to their normal karyotype and if it is malignant, the cells will continue to proliferate. Abbreviation: BM: bone marrow; SCL: sex chromosome loss; ACA: additional chromosome abnormality; LOY: loss of chromosome Y: LOX, loss of chromosome X.
Sex chromosome changes as a prognosis predictor in AML
LOY can often be observed with a myeloid malignancy like AML. Furthermore, LOY occurs as a primary cytogenetic abnormality as well as a secondary abnormality in AML patients, especially those with t(8,21) chromosomal abnormality, which could be associated with prognosis in these patients [Citation13,Citation14]. Studies have shown that LOX and LOY are observed in 30–40% and 50% of women and men with AML associated with t (8,21), respectively. t(8;21)(q22; q22) is a balanced reciprocal chromosomal abnormality that leads to the formation of AML/ETO fusion gene. The incidence of complex cytogenetic abnormalities in AML with t(8,21) is 9–23%, which has adverse effects on the prognosis of patients. Although LOX does not seem to be associated with a good prognosis in AML with t(8,21), LOY suggests a better prognosis in patients [Citation15,Citation16]. Other chromosomal abnormalities such as +4, +8, –9q, and tetraploidy can be associated with t(8,21) in rare cases, but their impact upon the prognosis of patients has not been clarified [Citation17]. Some studies have reported the presence of LOY in acute promyelocytic leukemia (APL) with t(15; 17)(q22; q21) and t(11; 17)(q23; q21), which indicates that LOY can be associated with AML with other translocations as well as t(8,21) [Citation18].
Derivative (der(Y) t(Y; 1)(q12; q21)) has been detected as a recurrent disorder and uncommon chromosomal rearrangement in AML. In addition, a case of t(Y; 11) with the involvement of MLL gene has been reported. Structural disorders like -Y are a rare occurrence in leukemia. der(Y) is observed only in a malignant cell clone and no inverted duplication has been reported so far to cause der(Y) [Citation19]. In AML with t(8; 21)(q22; q22), LOY can be developed after leukemia through the loss of CSF2RA gene that encodes the alpha subunit of GM-CSF receptor. If associated with t(8,21), molecular disorders, such as KIT and FLT-ITD mutations, can lead to poor prognosis in patients [Citation3,Citation15]. t(8; 21)(q22; q22) chromosomal abnormality has also been reported together with LOX, which is usually associated with a good prognosis () [Citation16].
In some studies, the men afflicted with AML showed LOY to a higher extent than men afflicted with myeloproliferative disease (MPD) or B-cell disorders. In these patients, the 45, X, -Y karyotype is probably caused by a malignant clone, even if no other cytogenetic disorder is observed in them. Although the LOY rate may change over time, the loss of this chromosome does not go together with disease progress. However, cytogenetic monitoring of patients can be an indication of disease progression, which requires further research in this area [Citation20]. Also, evaluation of the role of genes on sex chromosomes can be effective upon a better prediction of prognosis in AML patients ().
Sex chromosome changes as a rare abnormality in ALL
There are rare reports of a correlation between B-ALL and the loss of chromosome Y. The first report on the increased incidence of aneuploidy with age in human lymphocytes was published in 1970 [Citation3,Citation5]. However, LOY has been suggested to be more of an age-related phenomenon than a neoplastic one that is absent in B-ALL, since B-ALL is rather a primary disorder in children [Citation21]. ACA also occurs in ph+ B-ALL and is associated with a poor prognosis. However, LOY has not been reported as a secondary disorder in ph+ B-ALL yet. On the other hand, LOX has been reported in children with B-ALL and was associated with poor prognosis which can suggest that LOX is a neoplastic disorder than an age-related phenomenon in these patients [Citation22].
Although the Y chromosome mainly harbors the sex-linked genes, pseudoautosomal regions (PARs) are present on the chromosome Y similar to the X chromosome, which are inherited like other genes [Citation23]. Men harbor two alleles of these genes, and the question is whether the loss of these genes has a haploinsufficiency result or the mutations occurring in PAR regions of the X chromosome can play an oncogenic role in the absence of their homologous regions on the Y chromosome [Citation4].
After demonstrating the inhibitory role of the Y chromosome on a human prostate cancer cell line, it was hypothesized that the Y chromosome probably harbors a number of tumor suppressor genes. Recently, mutations and rearrangements in PAR1 genes, including cytokine receptor-like factor 2 (CRLF2) gene on Xp22.3/Yp11.3 chromosome, have been raised as an oncogene in over 7% of B-ALL cases [Citation24,Citation25]. However, the presence of these genes in PAR regions of X and Y chromosomes may represent their involvement in ALL. In addition, the mutation of these genes can address the presence of SCL as ACA in ALL patients, which is not just an age-related phenomenon. Overall, owing to the elusive role of sex chromosome loss in ALL, the assessment of oncogenes and tumor suppressor genes on these chromosomes could be associated with better prediction of pathogenesis process in this disorder ().
Sex chromosome changes in different phases of CML
In addition to classic t(9; 22) (q34; q11) in philadelphia chromosome, 3–5% of CML patients have ACA. CE is a criterion to define the accelerated phase in CML, which is associated with poor prognosis. LOY can, sometimes, be observed in ph+ patients as ACA or CE. However, it is difficult to determine the prognosis of patients with LOY [Citation2,Citation26]. Although CML is recognized by t(9; 22) (q34; q11) BCR-ABL1, ACA is usually manifested in the accelerated phase (AP) and the blast phase (BP). Despite the relationship between ACA and disease progression in CML, the role of ACA alone is unknown in this respect and a classification system similar to MDS and AML seems to be necessary for CML [Citation27]. In some studies, cytogenetic abnormalities in CML have been classified as major and minor. The former are common and include i(17) (q10), trisomy 8, and trisomy 19. The rest of the cases are uncommon and are associated with minor changes. This classification is based only on the frequency of ACAs and does not specify the prognosis or response to treatment in patients [Citation28,Citation29]. Chromosomal changes in CML during CE are highly heterogeneous, and it seems logical that different chromosomal changes cause a variety of changes in the disease process. Some changes simply reflect the genetic instability induced by BCR-ABL1, while others are indicative of disease progression and resistance to therapy [Citation30]. Although some studies indicate that major changes are associated with poor prognosis, others have shown that the major change of trisomy 8 has been associated with good prognosis and response to treatment. Notably, the association between this disorder and other chromosomal changes has led to poor prognosis in patients. Although some studies have given controversial results for a prognostic role of the Y chromosome in CML, these patients usually have a good prognosis [Citation31,Citation32].
However, LOY in association with t(9; 22) (q34; q11) in CML results in poor response to treatment. In patients treated with imatinib, LOY affects the likelihood and timing of cytogenetic and molecular changes as well as overall survival. These findings raised the question of whether -Y patients should undergo a similar treatment strategy with accelerated phase CML patients, which needs further investigations [Citation2,Citation3]. Overall, in addition to the need to classify cytogenetic abnormalities in CML, evaluation of the involved genes as well as BCR-ABL1 genes and their role in the pathogenesis in these patients seems to be necessary ().
Cytogenetic and molecular genetics of sex chromosome changes in MDS
Fifty percent of MDS cases show chromosomal abnormalities upon diagnosis, including –5q, –7q, –20q, +8 and LOY [Citation33,Citation34]. LOY is a common finding in MDS, which is seen in more than 15% of men with MDS. Patients only showing this cytogenetic abnormality, in general, show better prognosis and longer survival. Although this chromosomal abnormality affects the prognosis of MDS patients, it also occurs in older men with no history of hematologic malignancies. Recent studies indicated a significant correlation between LOY and MDS only in patients showing LOY in all their BM cells [Citation5,Citation35]. In any case, LOY by itself does not indicate the presence of a hematologic malignancy in patients lacking clinical and paraclinical symptoms of MDS. LOY is shown to be age-related and does not occur in MDS patients under 35 years, but is present in the cells of elderly men afflicted with MDS with or without clonal malignancy. However, the incidence of this disorder is higher in older MDS patients compared with healthy elderly men [Citation11]. The reason for this phenomenon can be genetic instability associated with age in all cell lines of these patients, which leads to disease progression in MDS patients. LOY often indicates the presence of an abnormal clone in myeloid cells in MDS. It is not known whether LOY causes this disease or not, but these cells may have other acquired genetic changes as well as LOY [Citation36,Citation37]. Studies assessing the occurrence of LOY in CD34+ cells in peripheral blood of subjects without hematologic malignancies have shown that the incidence of this chromosomal abnormality is not just indicative of MDS and can be due to BM failure caused by other factors or an aged BM [Citation38,Citation39]. However, the incidence of LOY in healthy people can be useful to predict clonal or non-clonal LOY. It has been recently shown that an increased risk of malignancy is higher in healthy subjects with LOY. Therefore, when associated with old age, LOY can anticipate the chance of other chromosomal abnormalities leading to malignancy in these people. However, a correct classification for LOY in different patients seems inevitable to understand the clonal nature of this disorder [Citation5,Citation40].
Abnormalities of the X chromosome are extremely rare in MDS. Isodicentric [idic(X)(q13)] has been reported in refractory anemia with ringed sideroblasts (RARS). Abnormalities in the X chromosome can be seen in RAEB and RAEB-t, which may confirm their role in the pathogenesis of MDS disease. In a molecular level, several genes have been identified on the X chromosome () [Citation9,Citation41,Citation42]. Chromosomal abnormalities, such as del (X)(q13) and del (X)(q24), indicate that the loss of specific genes in these regions may contribute to the pathogenesis of MDS. The genes involved in Xq13∼Xq24 rearrangements have been partially identified to date. The role of Braf2 genes in Xq13, API3 (XIAP) in Xq25, MYCL2 in Xq22∼q23 and LDOC1 in Xq27 in cell cycle regulation has been identified. However, recognizing the loss of these genes in a specific locus of the X chromosome, which is required to develop malignancy, is necessary and requires further investigations () [Citation12].
Sex chromosome changes as a clonal abnormality in CLL and MCL
Recently, the analysis of B- and T-cells in CLL patients has indicated that a large number of B-cells have SCL in contrast to T-cells [Citation11]. In general, a high percentage of cells with SCL among mononuclear cells or B-cells suggests that SCL is a clonal disorder of CLL cells. In -Y men with or without any hematologic malignancies, disregarding their age, if the percentage of -Y cells is >75%, it is likely to represent a clonal disorder [Citation20]. However, in recent studies, it has been shown that in some patients with CLL, despite the high rate of -Y in their cells, tumor infiltration in BM has been low, which is not indicative of a clonal disorder. Therefore, LOY may not be associated with disease progression in BM of these patients. When SCL is studied in various malignancies, it can indicate a primary or secondary change [Citation37]. In this theory, SCL can be an independent event not related to the development of leukemia. However, it can be raised that SCL represents a pre-leukemic phenotype exposing the cell to the development of acquired ACA. SCL can also secondarily occur in tumor cells during the malignancy process just like any other chromosomal disorder or possibly through the influence of age in BM progenitor cells ( and ) [Citation3,Citation20]. Secondary development of acquired SCL concomitant with karyotype changes in BM has been found in some malignancies. In mantle cell lymphoma (MCL), LOY is considered as a recurrent disorder secondary to t(11; 14) (q13; q32). Identification of a mutation in PAR1 and Xp22 as well as -Y leads to the loss of PAR1 and its associated genes, which demonstrates their role as tumor suppressor genes in MCL () [Citation43]. All these data show that SCL does not occur in tumor cells fortuitously, and can play an important role in the development of both myeloid and lymphoid disorders. Therefore, even if SCL is a function of old age, it can be a stimulus for CLL development in BM. Finally, although SCL is observed at a high rate in CLL and MCL cells, it is substantially associated with –13q. Therefore, SCL could be a clonal disorder in CLL and may contribute to the transformation of BM cells and leukemogenesis [Citation3]. Hence, the assessment of mutation or loss of genes that may be involved in the pathogenesis of CLL and MCL as well as the study of cytogenetic abnormalities can be helpful to improve the prediction of disease development process in these disorders ().
Table 1. Cytogenetic and molecular aberrations with SCL in leukemias.
Are sex chromosome changes in leukemia age-related or clonal abnormality?
Importance of the presence of 45, X, -Y cell population in leukemic patients as a marker of pathogenesis is not yet fully understood [Citation20]. That LOY is only a sign of normal aging phenomenon in the cell or indicative of an abnormal clone related to AML/MDS is difficult to understand due to the increased incidence of MDS and AML with age [Citation5,Citation55]. Studies have indicated a significant correlation between the risk of AML/MDS and that of LOY. When no cell with a natural karyotype is present in BM cytogenetic analysis, LOY may indicate the formation of a malignant clone in BM. Although complete LOY seems to be associated with AML/MDS, it is unlikely to cause these disorders by itself [Citation5]. Since LOY gradually occurs in some BM cells of men with an increase in their age, the presence of two cell populations with normal karyotype and LOY is a likely phenomenon [Citation5,Citation56]. AML/MDS is developed when secondary mutations occur in these cell lines. If this mutation randomly occurs in a cell that has lost its Y chromosome, the malignant cell will show 45, X, -Y karyotype and the proliferation of this clone will result in increased number of these cells relative to 46, XY cells in BM. This hypothesis confirms previous studies indicating the recovery of the normal karyotype in AML patients with LOY after treatment [Citation56] ( and ). LOY can also be seen in the complex karyotype and its association with +15 leads to a benign disorder in these patients. However, more studies are needed to fully assess the relationship between these disorders [Citation5,Citation57].
The mechanism of LOY in the induction or prognosis of leukemias is unclear. Few genes have been detected on the Y chromosome and none of them have been implicated in the progression of malignancies [Citation8]. Micro-HLA antigens encoded by genes on the Y chromosome have been shown to play an important role in the development of graft versus leukemia (GVL) following stem cell transplantation in ALL patients. LOY can potentiate the effect of GVL by increasing the probability of relapse in these patients. Therefore, LOY can be addressed as an acquired neoplastic disorder in B-ALL rather than being a purely age-related phenomenon [Citation4,Citation58]. Although we are unable to prove that the antigens associated with the Y chromosome increase the effect of GVL, considering the concurrence of LOY and relapse as well as the role of antigens associated with the Y chromosome as minor histocompatibility agents following PBSCT, it can be stated that the loss of GVL effect is due to the loss of the chromosome encoding these genes [Citation10]. With regard to the co-existence of both donor and recipient cells, the interpretation of SCL following allogeneic hematopoietic stem cell transplantation (alloHSCT) is dependent on multiple factors, including the age of donor and recipient, type of alloHSCT, the degree of chimerism, technical limitations of the methods used to detect SCL as well as other factors, which can be highly challenging. Testing for chimerism has been proved useful in determining the origin of SCL and clinical follow-up with a precise assessment of SCL by means of cytogenetic and molecular analysis is needed in these patients [Citation1]. Given the importance of LOY, comprehensive studies have been conducted concerning its role in various disorders, which indicate that over 75% of LOY cases are associated with hematologic diseases, which can significantly increase the risk of these malignancies [Citation20]. Interestingly, in patients with mosaic Turner syndrome, the presence of the Y chromosome is indicative of an increased risk of gonadoblastoma. LOY in patients with AML, which causes a more favorable prognosis, is an exception to this rule [Citation59].
Klinefelter syndrome (KS) with the classic form of 47, XXY is the most common sex chromosome disorder in men. However, the increase in sex chromosomes, including an increase in the X chromosome (over 4 chromosomes) and in the Y chromosome (over 2 chromosomes) as well as the mosaic form of 47, XXY/46, XY, has also been reported. KS is rarely associated with CML [Citation60–62]. In these patients, SCL occurs during the formation of abnormal karyotype, which leads to three different clones: 46, XX, -Y, t(9; 22)(q34; q11)/46, XX, t(Y; 20) (q11; q13), t (9; 22)(q34; q11), -der(Y) t(Y; 20) (q11; q13)/near-tetraploid (4n). Some studies confirm the association between the Y chromosome disorders in KS patients and CML in the formation of malignant clones with t(9,22) chromosomal abnormality [Citation7]. However, when LOY is, for the first time, observed in a patient referred for cytogenetic analysis, there is a big challenge in determining the prognosis and clinical course of the disease [Citation8].
Discussion and future perspective
LOY can act as an indirect marker to confirm the presence of a malignant clone, increased incidence of which raises the risk of leukemia development () [Citation5]. The importance of LOY as a marker for leukemias is not clear, and is believed to be related to unstimulated metaphases in BM of -Y men and no research has assessed LOX in leukemia up to now [Citation3]. LOY, when at least observed in metaphase cells, appears to be related to age. Conversely, when LOY is seen in the majority of cells, it is most likely a secondary event in CML, AML and MDS. In these circumstances, -Y can be used as a marker for genetic instability in patients with increased risk of malignancy or as an evidence for premature cellular aging in older people () [Citation56]. On the other hand, mutation or deletion in tumor suppressor genes and oncogenes in sex chromosomes during the aberration process will lead to the development of malignancy. Furthermore, disease prognosis and response to treatment will be different depending on the type of ACA that occurs concomitant with SCL in leukemic cells as well as the mutated or deleted gene () ( and ). Therefore, cytogenetic analysis and molecular changes in these leukemias can be suggested to be helpful for better prediction of prognosis as well as monitoring of the disease. But, still more studies are required to confirm this recommendation.
Acknowledgement
We thank all our colleagues in Golestan Hospital clinical research development unit, Ahvaz Jundishapur University of Medical Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Najmaldin Saki http://orcid.org/0000-0001-8494-5594
References
- Tang Z, Medeiros LJ, Yin CC, et al. Sex chromosome loss after allogeneic hematopoietic stem cell transplant in patients with hematologic neoplasms: a diagnostic dilemma for clinical cytogeneticists. Mol Cytogenet. 2016;9(1):141.
- Lippert E, Etienne G, Mozziconacci M-J, et al. Loss of the Y chromosome in Philadelphia-positive cells predicts a poor response of chronic myeloid leukemia patients to imatinibmesylate therapy. Haematologica. 2010;95(9):1604–1607. doi: 10.3324/haematol.2009.019109
- Chapiro E, Antony-Debre I, Marchay N, et al. Sex chromosome loss may represent a disease-associated clonal population in chronic lymphocytic leukemia. Genes Chromosome Canc. 2014;53(3):240–247. doi: 10.1002/gcc.22134
- Gupta A, Parihar M, Remani AS, et al. Loss of chromosome Y in acute lymphoblastic leukemia: age related or neoplastic phenomenon? Indian J Pathol Microbiol. 2014;57(3):431. doi: 10.4103/0377-4929.138742
- Wong AK, Fang B, Zhang L, et al. Loss of the Y chromosome: an age-related or clonal phenomenon in acute myelogenous leukemia/myelodysplastic syndrome? Arch Pathol Lab Med. 2008;132(8):1329–1332.
- Minner S, Kilgué A, Stahl P, et al. Y chromosome loss is a frequent early event in urothelial bladder cancer. Pathology. 2010;42(4):356–359. doi: 10.3109/00313021003767298
- Andreieva S, Korets K, Kyselova O, et al. Chronic myeloid leukemia in a patient with the Klinefelter syndrome. Exp Oncol. 2016;38(3):195–197.
- Zhang LJ, Shin ES, Yu ZX, et al. Molecular genetic evidence of Y chromosome loss in male patients with hematological disorders. Chin Med J. 2007;120(22):2002–2005.
- Michaux L, Wlodarska I, Mecucci C, et al. Characterization by chromosome painting of balanced and unbalanced X chromosome translocations in myelodysplastic syndromes. Cancer Genet Cytogenet. 1995;82(1):17–22. doi: 10.1016/0165-4608(94)00283-H
- Wolff D, Knopp A, Weirich V, et al. Loss of the GVL effect by loss of the Y-chromosome as putative mechanism of immune escape in ALL. Bone Marrow Transplant. 2005;35(1):101–102. doi: 10.1038/sj.bmt.1704712
- Ganster C, Kämpfe D, Jung K, et al. New data shed light on Y-loss-related pathogenesis in myelodysplastic syndromes. Genes Chromosome Canc. 2015;54(12):717–724. doi: 10.1002/gcc.22282
- Olshanskaya YV, Udovichenko AI, Vodinskaya LA, et al. Myelodysplastic syndromes with isolated deletion of the long arm of the chromosome X as a sole cytogenetic change. Cancer Genet Cytogenet. 2006;167(1):47–50. doi: 10.1016/j.cancergencyto.2005.08.018
- Shon W, Peters MS, Reed KB, et al. Atypical generalized eruptive histiocytosis clonally related to chronic myelomonocytic leukemia with loss of Y chromosome. J Cutan Pathol. 2013;40(8):725–729. doi: 10.1111/cup.12168
- Rowley JD. Recurring chromosome abnormalities in leukemia. Semin Hematol. 1990;27:122–136.
- Mohamed M, Dun K. Acute myeloid leukaemia with t(8; 21)(q22; q22. 3) and loss of the X chromosome. BMJ Case Reports. 2015.
- Kumari P, LingappaKavitha B, Reddy CO, et al. A rare cytogenetic presentation of acute myeloid leukemia (AML-M2). Acta Med Iran. 2012;50(12):827.
- Kuchenbauer F, Schnittger S, Look T, et al. Identification of additional cytogenetic and molecular genetic abnormalities in acute myeloid leukaemia with t (8; 21)/AML1-ETO. Br J Haematol. 2006;134(6):616–619. doi: 10.1111/j.1365-2141.2006.06229.x
- Wu Y, Xue Y, Pan J. Y-chromosome loss in acute promyelocytic leukemia. Cancer Genet Cytogenet. 2005;157(1):90–91. doi: 10.1016/j.cancergencyto.2004.04.022
- Othman MA, Vujić D, Zecević Z, et al. A cryptic three-way translocation t (10; 19; 11)(p12. 31; q13. 31; q23. 3) with a derivative Y-chromosome in an infant with acute myeloblastic leukemia (M5b). Gene. 2015;563:115–119. doi: 10.1016/j.gene.2015.02.064
- Wiktor A, Rybicki BA, Piao ZS, et al. Clinical significance of Y chromosome loss in hematologic disease. Genes Chromosome Canc. 2000;27(1):11–16. doi: 10.1002/(SICI)1098-2264(200001)27:1<11::AID-GCC2>3.0.CO;2-I
- Abeliovich D, Yehuda O, Ben-Neriah S, et al. Loss of Y chromosome. An age-related event or a cytogenetic marker of a malignant clone? Cancer Genet Cytogenet. 1994;76:70–71. doi: 10.1016/0165-4608(94)90075-2
- Settin A, Al Haggar M, Al Dosok T, et al. Prognostic cytogenetic markers in childhood acute lymphoblastic leukemia: cases from Mansoura, Egypt. Hematology. 2006;11(5–6):341–349. doi: 10.1080/10245330600938174
- Heerema NA, Harbott J, Galimberti S, et al. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia. 2004;18:693–702. doi: 10.1038/sj.leu.2403324
- Vijayakumar S, Garcia D, Hensel CH, et al. The human Y chromosome suppresses the tumorigenicity of PC-3, a human prostate cancer cell line, in athymic nude mice. Genes Chromosome Canc. 2005;44:365–372. doi: 10.1002/gcc.20250
- Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397
- Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk" chronic granulocytic leukemia. Blood. 1984;63(4):789–799.
- Wang W, Cortes JE, Tang G, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127(22):2742–2750. doi: 10.1182/blood-2016-01-690230
- Baccarani M, Deininger MW, Rosti G, et al. European leukemianet recommendations for the management of chronic myeloid leukemia. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569
- Perrotti D, Jamieson C, Goldman J, et al. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264. doi: 10.1172/JCI41246
- Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7(6):441–453. doi: 10.1038/nrc2147
- Luatti S, Castagnetti F, Marzocchi G, et al. Additional chromosomal abnormalities in Philadelphia-positive clone: adverse prognostic influence on frontline imatinib therapy: a GIMEMA working party on CML analysis. Blood. 2012;120(4):761–767. doi: 10.1182/blood-2011-10-384651
- Wang W, Cortes JE, Lin P, et al. Impact of trisomy 8 on treatment response and survival of patients with chronic myelogenous leukemia in the era of tyrosine kinase inhibitors. Leukemia. 2015;29(11):2263–2266. doi: 10.1038/leu.2015.96
- Mallo M, Luño E, Sanzo C, et al. Clinical impact of the clone size in MDS cases with monosomy 7 or 7q deletion, trisomy 8, 20q deletion and loss of Y chromosome. Leuk Res. 2011;35(6):834–836. doi: 10.1016/j.leukres.2011.01.003
- Cogle CR, Saki N, Khodadi E, et al. Bone marrow niche in the myelodysplastic syndromes. Leuk Res. 2015;39(10):1020–1027. doi: 10.1016/j.leukres.2015.06.017
- Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–4395. doi: 10.1182/blood-2007-03-082404
- Boultwood J, Wainscoat JS. Clonality in the myelodysplastic syndromes. Int J Hematol. 2001;73:411–415. doi: 10.1007/BF02994002
- Wiktor AE, Van Dyke DL, Hodnefield JM, et al. The significance of isolated Y chromosome loss in bone marrow metaphase cells from males over age 50 years. Leuk Res. 2011;35:1297–1300. doi: 10.1016/j.leukres.2011.05.002
- Vehmeyer K, Haase D, Alves F. Increased peripheral stem cell pool in MDS: an indication of disease progression? Leuk Res. 2001;25:955–959. doi: 10.1016/S0145-2126(01)00064-9
- Braulke F, Jung K, Schanz J. Molecular cytogenetic monitoring from CD341 peripheral blood cells in myelodysplastic syndromes – first results from a prospective multicenter German diagnostic study. Leuk Res. 2013;37:900–906. doi: 10.1016/j.leukres.2013.03.019
- Forsberg LA, Rasi C, Malmqvist N, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46:624–628. doi: 10.1038/ng.2966
- Knight JC, Grimaldi G, Thiesen H-J, et al. Clustered organization of Krfippel zinc-finger genes at Xp11.23, flanking a translocation breakpoint at OATLI: a physical map with locus assignment for ZNF21, ZNF41, ZNF81, and ELK1. Genomics. 1994;21:180–187. doi: 10.1006/geno.1994.1240
- de Leeuw B, Suikerbuijk RF, OldeWeghuis D, et al. Distinct Xp11.2 breakpoint regions in synovial sarcoma revealed by metaphase and interphase FISH: relationship to histologic subtypes. Cancer Genet Cytogenet. 1994;73:89–94. doi: 10.1016/0165-4608(94)90191-0
- Nielander I, Martin-Subero JI, Wagner F, et al. Recurrent loss of the Y chromosome and homozygous deletions within the pseudoautosomal region 1: association with male predominance in mantle cell lymphoma. Haematologica. 2008;93:949–950. doi: 10.3324/haematol.12656
- Weng S, Stoner S, Zhang D. Sex chromosome loss and the pseudoautosomal region genes in hematological malignancies. Oncotarget. 2016;7(44):72356–72372. doi: 10.18632/oncotarget.12050
- Testa U, Riccioni R, Diverio D, et al. Interleukin-3 receptor in acute leukemia. Leukemia. 2004;18(2):219–226. doi: 10.1038/sj.leu.2403224
- Hruz T, Laule O, Szabo G, et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform. 2008;2008:1–5. doi: 10.1155/2008/420747
- Hornakova T, Staerk J, Royer Y, et al. Acute lymphoblastic leukemia-associated JAK1 mutants activate the Janus kinase/STAT pathway via interleukin-9 receptor alpha homodimers. J Biol Chem. 2009;284(11):6773–6781. doi: 10.1074/jbc.M807531200
- Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci USA. 2010;107(45):19455–19460. doi: 10.1073/pnas.1008271107
- Dworzak MN, Fröschl G, Printz D, et al. CD99 expression in T-lineage ALL: implications for flow cytometric detection of minimal residual disease. Leukemia. 2004;18(4):703–708. doi: 10.1038/sj.leu.2403303
- Büyükavci M, Ozdemir O, Buck S, et al. Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam Clin Pharmacol. 2006;20(1):73–79. doi: 10.1111/j.1472-8206.2005.00389.x
- Zhang J, Mullighan CG, Harvey RC, et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: a report from the children’s oncology group. Blood. 2011;118(11):3080–3087. doi: 10.1182/blood-2011-03-341412
- Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130(1):21–24. doi: 10.1016/j.cell.2007.06.034
- Yang Z, Cheng W, Hong L, et al. Adenine nucleotide (ADP/ATP) translocase 3 participates in the tumor necrosis factor induced apoptosis of MCF-7 cells. Mol Biol Cell. 2007;18(11):4681–4689. doi: 10.1091/mbc.E06-12-1161
- Nieländer I, Martín-Subero JI, Wagner F, et al. Recurrent loss of the Y chromosome and homozygous deletions within the pseudoautosomal region 1: association with male predominance in mantle cell lymphoma. Haematologica. 2008;93(6):949–950. doi: 10.3324/haematol.12656
- United Kingdom Cancer Cytogenetics Group (UKCCG). Loss of the Y chromosome from normal and neoplastic and bone marrows. Genes Chromosome Canc. 1992;5:83–88. doi: 10.1002/gcc.2870050112
- Riske CB, Morgan R, Ondreyco S, et al. X and Y chromosome loss as sole abnormality in acute non-lymphocytic leukemia (ANLL). Cancer Genet Cytogenet. 1994;72:44–47. doi: 10.1016/0165-4608(94)90108-2
- Herens C, Brasseur E, Jamar M, et al. Loss of the Y chromosome in bone marrow cells: results on 1907 consecutive cases of leukemia and proleukemia. Clin Lab Haem. 1999;21:17–20. doi: 10.1046/j.1365-2257.1999.00173.x
- Randolph SS, Gooley TA, Warren EH, et al. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347–352. doi: 10.1182/blood-2003-07-2603
- Aly MS, Khaled HM. Chromosomal aberrations in early-stage bilharzial bladder cancer. Cancer Genet Cytogenet. 2002;132:41–45. doi: 10.1016/S0165-4608(01)00527-1
- Toubai T, Tanaka J, Ota S, et al. Allogeneic bone marrow transplantation from an unrelated donor for the treatment of chronic myelogenous leukemia in blast crisis in a patient with Klinefelter’s syndrome. Leuk Lymphoma. 2004;45:829–831. doi: 10.1080/10428190310001607188
- Chennuri V, Kashyap R, Tamhankar P, et al. Chronic myeloid leukemia in case of Klinefeltersyndrome. Indian J Hum Genet. 2014;20:69–71. doi: 10.4103/0971-6866.132760
- Chakraborty R, Mukkamalla SKR, Singam K, et al. Persistent suboptimal molecular response in a patient with chronic myelogenous leukemia and Klinefelter syndrome. Korean J Intern Med. 2014;29:827–829. doi: 10.3904/kjim.2014.29.6.827
