ABSTRACT
Objective: To estimate the associations between HLA-A/B/DRB1 polymorphisms and aplastic anemia (AA), we carried out the meta-analysis.
Methods: In this meta-analysis, all publications in English and Chinese were considered up to 30 September 2015. The electronic databases we searched were Pubmed, Science Direct, Embase, Web of Science, CNKI, Wanfang Data and VIP. We conducted all statistical data analyses in the Stata11.0 software.
Results: A total of 17 studies including 9164 subjects (containing 1372 cases and 7792 controls) were retrieved, which studied the relationship between HLA-A/B/DRB1 and AA. Odds ratios (ORs) with 95% confidence intervals (CIs) for the comparisons between cases and controls were calculated. The result revealed that HLA-A*02 and HLA-DRB1 (*0407, *15 and *1501) polymorphisms might increase the risk of AA. Otherwise, HLA-DRB1 (*0301, *04, *0406, *0802, *1301, *1302 and *14) were protective against AA. But, other sites of HLA-A/B/DRB1 in our study had no correlations with AA (all Pc > 0.05).
Conclusion: In conclusion, HLA-A/B/DRB1 polymorphisms may play an important role in AA, but higher quality and larger sample studies are needed to confirm.
Introduction
Aplastic anemia (AA) is a hematological disease with the characteristics of pancytopenia in peripheral blood and hypoplasia in bone marrows [Citation1,Citation2]. The incidence rates in Europe and the United States are 0.8 and 1.5 new cases per million, respectively, two or three times that in Asia [Citation3–5]. The etiology is unclear, but many studies have reported that genetic and environmental factors were the crucial determinants of susceptibility to AA [Citation1,Citation2,Citation6]. Meanwhile, some studies have indicated that genetic components of the immune response and human leukocyte antigen (HLA) system have a positive correlation in different ethnic groups in particular [Citation7,Citation8].
HLA genes are found in 6p2.13 and are considered to be one of the most polymorphic genes, which play a key role in regulating the human immune response [Citation9,Citation10]. HLA included 224 genes loci, 128 of which are functional genes, 40% of which are related to immune system [Citation11]. The classical HLA genes are divided into class I (HLA-A, HLA-B, HLA-C, etc.) and class II (HLA-DR, HLA-DQ, HLA-DP, etc.) genes [Citation11]. The HLA-I loci encode the endogenous antigen molecules, present them to CD8 + T cells, and initiate cytotoxic T-cell response. The HLA-II loci bind exogenous antigen peptides, present them to CD4+ T cells, mediate cytokine production, and help in antibody production [Citation12]. Activated cytotoxic T lymphocytes induce the Fas-mediated apoptosis of the hematopoietic stem cells (HSCs) and produce IFN-γ that can interrupt the cell cycle, thereby suppressing hematopoiesis [Citation13,Citation14].
Although the pathogenesis of AA is not well known, some studies have reported that it is greatly related to the immune system [Citation8,Citation15]. For example, Sugimori et al. [Citation16] suggested that oligoclonal cytotoxic T-cells induce immune responses, which targeted on hematopoietic stem cells and progenitor cells, leading to cell apoptosis and marrow failure. In addition, accumulating evidence showed that it is an autoimmune disease caused by the T-cell-mediated destruction of hematopoietic stem and progenitor cells, thereby leading to the bone marrow suppression [Citation17].
Recently, many studies have focused on the relationship between HLA polymorphism and AA. Moreover, numerous studies showed that HLA-A, HLA-B and HLA-DRB1 were closely associated with susceptibility to AA, but still there is a lack of consistent result. Some studies have been conducted to investigate the association between HLA-DRB1*04, *14, *15 polymorphism and AA [Citation1,Citation4,Citation9,Citation18,Citation19]; however, the results have generated considerable controversy. Interestingly, one study reported that HLA-DRB1*0301 played a protective role in AA [Citation20], but some studies suggested that it has no association with AA [Citation2,Citation7,Citation8,Citation21]. When Dhaliwal et al. [Citation20] and Liang et al. [Citation21] considered that HLA-DRB1*1501 had no relationship with AA, two studies manifested that HLA-DRB1*1501 is susceptible to it [Citation2,Citation8]. In addition, three articles [Citation2,Citation22,Citation23] indicated that HLA-A*2 polymorphism was unrelated to AA, while one study [Citation24] supported the association between HLA-A*02 polymorphism and AA. So, we performed this study to better clarify the association between the HLA polymorphism and AA. To the best of our knowledge, it is the first meta-analysis of the association between HLA polymorphism and AA.
Methods
Search strategy
All publications in English and Chinese were considered up to 30 September 2015. The electronic databases we searched were Pubmed, Science Direct, Embase, Web of Science, CNKI, Wanfang Data and VIP. The search strategy was based on a combination of the keywords (‘HLA-DRB1’ OR ‘HLA-A’ OR ‘HLA-B’ OR ‘HLA’ OR ‘human leukocyte antigen’) AND (‘polymorphism’ OR ‘mut*’ OR ‘varia*’) AND (‘aplastic anemia’ OR ‘AA’). Moreover, the reference lists of all retrieved publications were manually searched for acquiring the relevant publications. And if the additional sources of relevant research were needed, we contacted the authors by email or by other ways.
Selection criteria
All the studies included in the meta-analysis conforming to the inclusion criteria are as follows: (1) case–control studies which assess the association between HLA-A/B/DRB1 and AA; (2) original research; (3) offered sufficient data to calculate an OR with 95% CI; (4) the studies for each gene loci at least three posts; (5) language restriction was English or Chinese. Furthermore, exclusion criteria included: (1) repetition of the study; (2) studies that cannot be obtained; (3) animal studies.
Data extraction
The information extracted from the selected studies included: first author (year), country, region, the numbers of cases and controls, genotyping methods, source of controls, diagnostic criteria of cases, genotype. Two investigators (Du and Deng) extracted data in accordance with the inclusion/exclusion criteria. If there was discordance, a third investigator (Peng) was inducted to reach agreement on all items.
Statistical analysis
We conducted all statistical data analyses in the Stata11.0 software. The strength of association between HLA-A/B/DRB1 and AA was measured by calculating pooled ORs with corresponding 95% CIs. In addition, the Cochran’s Q test (α = 0.05) and Higgins’s (I2 ) tests were performed to assess the heterogeneity in all studies. We used the fixed effect-model for meta-analysis when the heterogeneity was not statistically significant (I2 < 50%, or p > 0.05), otherwise, a random-effect model was applied. What is more, publication bias was estimated by the method of Egger’s test.
Results
Search results
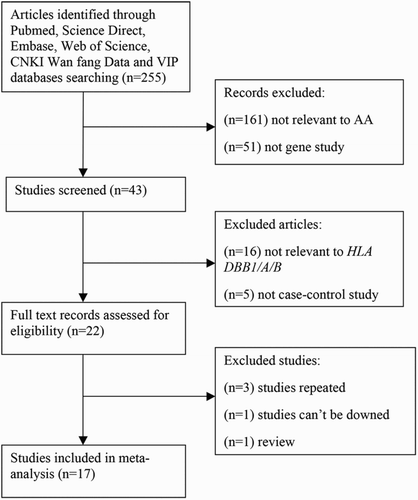
As shown in , 255 articles were identified by initial published work search. By reviewing the titles and abstracts of the initial publications, we acquired 22 applicable studies. After screening the full text, five studies were excluded based on the selection criteria. Of these excluded studies, three studies were repeated, one study was a review, and the sufficient data of one record cannot be obtained even if we contacted author by email or other ways. Ultimately, we adopted 17 articles in our meta-analysis to analyze the associations between HLA-A/B/DRB1 and AA [Citation1,Citation2,Citation8,Citation13,Citation16,Citation18–29].
Study characteristics
In , a total of 17 studies including 9164 subjects (containing 1372 cases and 7792 controls) were retrieved, which studied the relationship between HLA-A/B/DRB1 and AA. All of the eligible studies, twelve studies were related to Asia, two studies related to North America, and three studies related to Europe. In terms of source of controls, six studies were derived from hospital, ten from population, and one not mentioned. Polymerase chain reaction (PCR) was used to make sure the genotyping for 14 studies, one was typed by the standard two-step NIH Terasaki microlymphocytotoxicity test, and one was not mentioned.
Table 1. Characteristics of the studies included in the meta-analysis.
Association between HLA-A and AA
In our meta-analysis, five loci in were researched for the relationship between HLA-A and AA, and each locus had four studies. However, only the HLA-A*02 had statistically significant relationship with AA, and HLA-A*02 was considered to be a risk factor (OR = 1.377, Pc = 0.010, 95% CI: 1.081–1.754, ) with no evidence of heterogeneity (I2 = 0.0%).
Figure 2. Forest plots of HLA-A*02 which had statistically significant relationship with aplastic anemia.
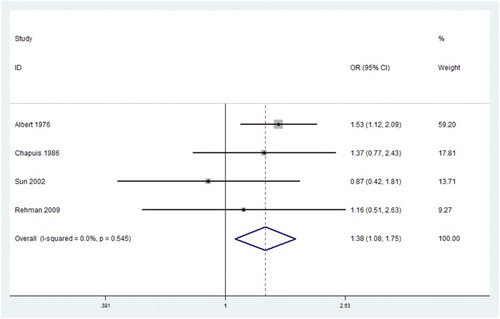
Table 2. Associations between the HLA-A polymorphism and aplastic anemia.
Association between HLA-B and AA
In , a total of 10 loci were about HLA-B and each of them had three to five related studies, but none of them (HLA-B*3501, *05, *07, *08, *12, *13, *14, *18, *27, *40) had significant connection with AA ().
Table 3. Associations between the HLA-B polymorphism and aplastic anemia.
Association between HLA-DRB1 and AA
In , there were about three to six studies for each locus about the association between HLA-DRB1 and AA. Ten loci were significantly related to AA, but the others were not. Among these loci, HLA-DRB1 (*0407, *15, *1501) were the risk factors for AA, and the pooled ORs and 95% CIs of them were 3.741(1.009–12.734), 2.997 (1.748–5.137), and 2.234 (1.491–3.349). On the contrary, we found that HLA-DRB1 (*0301, *04,*0406,*0802, *1301, *1302, *14) were protective for AA, the pooled ORs and 95% CIs of them were 0.565 (0.335–0.953), 0.473 (0.257–0.872), 0.392 (0.214–0.716), 0.454 (0.220–0.937), 0.325 (0.125–0.845), 0.593 (0.392–0.897), and 0.530 (0.320–0.877), and none of them showed significant heterogeneity(I2 < 50.0%).
Table 4. Summary results for meta-analysis of HLA-DRB1 polymorphism and aplastic anemia.
Publication bias
Publication bias was assessed with the Egger’s test. No publication bias was found in the meta-analysis, except for HLA-DRB1*01, *1502 and *0801 (p < 0.05).
Subgroup analysis
In our study, more than or equal to four articles of the site, we performed the subgroup analysis. The results showed that HLA-A*02 was not associated with AA in the Asian population and was a risk factor for AA in non-Asian. In contrast, HLA-DRB1*14 is a protective factor for AA in Asia and is not associated with the incidence of AA in non-Asian populations. HLA-DRB1*04 had no effect on the incidence of AA in Asia and non-Asia. HLA-DRB1*15 can promote the occurrence of AA in Asia and non-Asia. The data of HLA-DRB1 (*0301, *0406, *0802, *1301, *1302, *0407, and *1501, etc.) sites were all from Asia, so we cannot clarify the impact of these sites on AA in non-Asian populations. In the HLA-A*10/B*13/DRB1*03 locus, when we combined the data, it was not statistically significant. In the subgroup analysis, it showed that HLA-A*10 can promote the occurrence of AA in Asia and has no effect on AA in non-Asia. HLA-B*13 is not associated with the occurrence of AA in Asia, but it is a predisposing factor for AA in non-Asia. HLA-DRB1*03 can reduce the incidence of AA in Asia, while it has nothing to do with AA in non-Asia. HLA-DRB1*07/DRB1*11/DRB1*13, etc., loci were not associated with AA in Asia or non-Asia or the total population ().
Table 5. The outcomes of the subgroup analysis.
Discussion
As far as we know, the current qualitative systematic or meta-analytic review had not provided an extended and comprehensive overview of the correlations between HLA-A/B/DRB1 polymorphisms and the risk of AA. We discovered that 11 loci of HLA alleles were distinctly associated with AA in total. Four of those variants including HLA-A*02 and HLA-DRB1 (*0407, *15 and *1501) were the significant risk factors for AA. But, HLA-DRB1 allele subtypes (*0301, *04, *0406, *0802, *1301, *1302 and *14) acted as protective factors against AA.
Although the exact pathophysiology of AA is still indistinct, it is associated with hematopoietic stem/progenitor cell (HSC/HPC) abnormalities, hematopoietic microenvironmental dysfunction, immune mechanism abnormalities and changes in gene levels. First, HSC/HPC plays a decisive role in maintaining hematopoiesis. And the mechanism of hematopoietic stem / progenitor cell abnormalities is as follows, Cytotoxic cells directly kill HSC through cytotoxicity and hematopoietic inhibitory factor through the current recognized Fas / FasL and NO pathway to HSC apoptosis [Citation31]. Second, hematopoietic microenvironment defects or abnormalities induced AA. Bone marrow mesenchymal stem cells (BMSCs) are important stem cells in hematopoietic microenvironment. Normal BMSCs inhibit T-cell activity and provide bone marrow hematopoietic stem cells a microenvironment with immunoprotective effects. AA patients with BMSC morphology were abnormal, the proliferation of BMSC morphology is not high, increased apoptosis and easily differentiated into adipocytes, which induced disease [Citation32]. Third, abnormal immune mechanisms can also lead to AA. Studies have found that the number of AA patients with the peripheral blood lymphocytes and NK cells significantly increased, and after immunotherapy, the percentage decreased significantly. The expression rates of NKp46, NKG2D and CD158a were significantly higher in NK cells in AA patients than in normal cells, and the expression level of perforin was also higher [Citation33], which are likely to induce T lymphocyte hyperthyroidism, resulting in AA patients with hematopoietic failure [Citation34]. Fourth, the telomerase RNA component of the AA patient or the telomerase reverse transcription (TERT) gene mutation and the chromosome terminal rate decreased, leading to differentiation defects in telomerase mutation . At the same time, AA patients had hematopoietic TERC and TERT gene mutations, telomere-associated protein gene abnormal expression, and telomere protective protein was significantly reduced [Citation35]. To sum up, we believe that AA is an autoimmune disease; its pathogenesis is a variety of immune mechanisms and genetic changes in the role of synergies. Therefore, our study will have a great significance in exploring the pathogenesis of AA.
Our article, from the genetic point of view, explains the pathogenesis of AA, the understanding of which may play an important role in the treatment and prevention of AA. In 2016, Liu et al. [Citation17] showed that for the AA patients in Asia, immunosuppressive therapy (IST) may be more effective for HLA-DRB1*15+ and HLA-DRB1*15:01+ than HLA-DRB*15− and HLA-DRB1*15:01−.
Our data confirmed that HLA-A*02 and HLA-DRB1 (*0407, *15 and *1501) were the risk factors for AA and the results were the same as the studies [Citation1,Citation25,Citation36,Citation37]. A study showed that HLA-DRB1*15 and *1501 were significantly higher in AA patients than the control group [Citation16]. Wang et al. [Citation2] found that HLA-DRB1*1501 significantly increased in SAA patients. Several research studies found a drastically elevated quantity of HLA-DRB1*1501 gene frequency with AA patients [Citation2,Citation16,Citation18,Citation20], indicating that HLA-DRB1*1501 allele might contribute to AA. Moreover, our data showed that HLA-DRB1*0407 allele was a risk-conferring allele, but previous studies failed to show the association between HLA-DRB1*0407 and AA [Citation2,Citation16,Citation18]. The data of Wang et al. [Citation2], Dhaliwal et al. [Citation20], Rehman et al. [Citation1] and Sugimori et al. [Citation16] reported that some sites of HLA-A*02 elevated in patients with severe AA which inferred an association of HLA-A*02 subtypes with AA. However, the correlation between HLA-A*02 and AA could not be clarified in other previous studies [Citation1,Citation19,Citation22,Citation26,Citation28].
HLA-DRB1 allele subtypes (*0301, *04, *0406, *0802, *1301, *1302 and *14) were found to be the protective factors against AA. We detected that HLA-DRB1*0301 decreased the susceptibility to AA. Nevertheless, the correlation had only been reported among child patients with AA [Citation15,Citation22]. Additionally, four studies failed to demonstrate the association of HLA-DRB1*0301 with AA [Citation2,Citation16,Citation18,Citation19]. The data of the associations between HLA-DRB1 *1301, *1302 alleles and AA from these previous studies were inconsistent [Citation2,Citation4,Citation18,Citation21]. Liang et al. [Citation21] approved of a negative correlation between HLA-DRB1*1301 and AA. The study carried out by Song et al. [Citation18] indicated that HLA-DRB1*1302 played its protective role only in adults, while other studies [Citation2,Citation4] failed to confirm the differences between AA patients and controls. The data from these reports were inconsistent; we, therefore, combined quantitative evidence from these studies to offer direct support for the conclusion that HLA-DRB1 (*1301 and *1302) allele reduced the chance of suffering from AA and HLA-A*02 allele improved the incidence of AA.
Moreover, we also presented that HLA-DRB1*14 could decrease the disposition of AA in conformity to Huo’s study [Citation19], whereas some studies confirmed the conclusion abortively [Citation1,Citation16,Citation25,Citation36]. This may be due to the low frequency of HLA-DRB1 (*0407, *0802,*0301 and *14) in human and small sample sizes of previous studies. Therefore, further studies involving a larger number of patients and controls are needed to clarify this point. Kapustin et al. [Citation14] noted that HLA-DRB1*04 subtypes led to the progression of AA. On the contrary, many studies supported the point that HLA-DRB1*04 played a protective role in AA [Citation8,Citation38]. What is more, a significantly protective association between HLA-DRB1*0406 and AA was shown in the current study. In contrast, previous studies [Citation2,Citation16,Citation18] were unable to define the relationship between HLA-DRB1*0406 and AA. The mechanism by which HLA-DRB1*04 subtypes alleles polymorphisms develop and affect the progress of AA is still indistinct. As our data showed, HLA-DRB1*04 can decrease the incidence of AA: however, HLA-DRB1*0407 is a potential risk factor of AA which has an opposite effect with HLA-DRB1*0406. This result is a real puzzled question and further investigations need to be performed to explore the exact pathogenesis.
The data from these reports were inconsistent, we, therefore, combined quantitative evidence from these studies to further confirm the association between HLA-A/B/DRB1 polymorphisms and AA. It is worth mentioning that in addition to the existence of heterogeneity in HLA-DRB1 (*04, *15 and *1501) sites, the I2 in the other statistical significance of the site is less than 50%, indicating that the data of the heterogeneity are relatively small, which can be combined with statistics, so our results can be trusted to a certain degree.
Some limitations in our study should be noted in order to interpret the results better. First, all included studies in this meta-analysis were mainly derived from the databases of English and Chinese, might lead to a potential language bias. Second, our meta-analysis only involved 17 studies which contained 1372 AA cases and 7792 controls. The small sample sizes may limit the statistical power. Third, the diagnostic standard of AA is inconsistent in the included studies, which may lead to select bias. Fourth, the assessment among gene–gene and gene–environment and other potential interactions may affect the sensibility of AA. Fourth, our study focussed on all types of AA, while some of the included studies only focus on SAA. Fifth, although most of the loci, we researched, did not show significantly heterogeneous, some loci showed heterogeneity.
In conclusion, a link between HLA-A/B/DRB1 allele polymorphisms and AA was demonstrated in the meta-analysis. Our finding illuminated that HLA-A*02, HLA-DRB1*0407, *15 and *1501 were risk factors for AA, while HLA-DRB1*0301, *04, *0406, *0802, *1301, *1302 and *14 alleles were the protective factors. Further investigations need to be performed for better demonstrating the relationship between HLA-A/B/DRB1alleles polymorphisms and AA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rehman S, Saba N, Khalilullah Munir S, et al. The frequency of HLA class I and II alleles in Pakistani patients with aplastic anemia. Immunol Invest. 2009;38(8):812–819. DOI:10.3109/08820130903271415.
- Wang M, Nie N, Feng S, et al. The polymorphisms of human leukocyte antigen loci may contribute to the susceptibility and severity of severe aplastic anemia in Chinese patients. Hum Immunol. 2014;75(8):867–872. DOI:10.1016/j.humimm.2014.06.011.
- Benitez-Aranda H, Velez-Ruelas MA, Diaz-Cardenas S, et al. Incidence of aplastic anemia in a defined subpopulation from Mexico city. Hematology. 2002;7(4):229–232. DOI:10.1080/1024533021000024085.
- Marsh JC, Ball SE, Darbyshire P, et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol. 2003;123(5):782–801. doi: 10.1046/j.1365-2141.2003.04721.x
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 2006;108(8):2509–2519. DOI:10.1182/blood-2006-03-010777.
- Shao W, Tian D, Liu C, et al. Aplastic anemia is associated with HLA-DRB1*1501 in northern Han Chinese. Int J Hematol. 2000;71(4):350–352.
- Kordasti S, Marsh J, Al-Khan S, et al. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119(9):2033–2043. DOI:10.1182/blood-2011-08-368308.
- Yari F, Sobhani M, Vaziri MZ, et al. Association of aplastic anaemia and Fanconi's disease with HLA-DRB1 alleles. Int J Immunogenet. 2008;35(6):453–456. DOI:10.1111/j.1744-313X.2008.00810.x.
- Hughes AL, Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988;335(6186):167–170. doi:10.1038/335167a0.
- Hughes AL, Nei M. Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Natl Acad Sci USA. 1989;86(3):958–962. doi: 10.1073/pnas.86.3.958
- Complete Sequence and Gene Map of a Human Major Histocompatibility Complex. The MHC sequencing consortium. Nature. 1999;401(6756):921–923. DOI:10.1038/44853.
- Schultz JC, Shahidi NT. Detection of tumor necrosis factor-alpha in bone marrow plasma and peripheral blood plasma from patients with aplastic anemia. Am J Hematol. 1994;45(1):32–38. doi: 10.1002/ajh.2830450106
- Shichishima T, Noji H, Ikeda K, et al. The frequency of HLA class I alleles in Japanese patients with bone marrow failure. Haematologica. 2006;91(6):856–857.
- Kapustin SI, Popova TI, Lyshchov AA, et al. HLA-DR4-Ala74 beta is associated with risk and poor outcome of severe aplastic anemia. Ann Hematol. 2001;80(2):66–71. doi: 10.1007/s002770000234
- Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15(3):162–168. DOI:10.1097/MOH.0b013e3282fa7470.
- Sugimori C, Yamazaki H, Feng X, et al. Roles of DRB1 *1501 and DRB1 *1502 in the pathogenesis of aplastic anemia. Exp Hematol. 2007;35(1):13–20. DOI:10.1016/j.exphem.2006.09.002.
- Liu S, Li Q, Zhang Y, et al. Association of human leukocyte antigen DRB1*15 and DRB1*15:01 polymorphisms with response to immunosuppressive therapy in patients with aplastic anemia: a meta-analysis. PLoS One. 2016;11(9):e0162382. DOI:10.1371/journal.pone.0162382.
- Song EY, Park S, Lee DS, et al. Association of human leukocyte antigen-DRB1 alleles with disease susceptibility and severity of aplastic anemia in Korean patients. Hum Immunol. 2008;69(6):354–359. DOI:10.1016/j.humimm.2008.04.009.
- Huo MR, Yu Y, Liu HY, et al. Association of HLA DRB1 polymorphism with susceptibility to myelodysplastic syndrome and aplastic anemia in Chinese Han population. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011;28(3):296–299. DOI:10.3760/cma.j.issn.1003-9406.2011.03.013.
- Dhaliwal JS, Wong L, Kamaluddin MA, et al. Susceptibility to aplastic anemia is associated with HLA-DRB1*1501 in an aboriginal population in Sabah, Malaysia. Hum Immunol. 2011;72(10):889–892. DOI:10.1016/j.humimm.2011.06.013.
- Liang XL, Qiu LG, Sun LJ, et al. Correlation of HLA-alleles with aplastic anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15(6):1208–1211.
- Chapuis B, Von Fliedner VE, Jeannet M, et al. Increased frequency of DR2 in patients with aplastic anaemia and increased DR sharing in their parents. Br J Haematol. 1986;63(1):51–57. doi: 10.1111/j.1365-2141.1986.tb07494.x
- Sun W, Zhao S. Study on the association of TCM syndrome differentiation of chronic aplastic anemia and HLA gene. China J Tradit Chin Med Pharm. 2002;1:167–169.
- Albert E, Thomas ED, Nisperos B, et al. HLA antigens and haplotypes in 200 patients with aplastic anemia. Transplantation. 1976;22(5):528–531. doi: 10.1097/00007890-197611000-00020
- Fernandez-Torres J, Flores-Jimenez D, Arroyo-Perez A, et al. The ancestry of the HLA-DRB1*15 allele predisposes the Mexican mestizo to the development of aplastic anemia. Hum Immunol. 2012;73(8):840–843. DOI:10.1016/j.humimm.2012.04.012.
- Chen C, Lu S, Luo M, et al. Correlations between HLA-A, HLA-B and HLA-DRB1 allele polymorphisms and childhood susceptibility to acquired aplastic anemia. Acta Haematol. 2012;128(1):23–27. DOI:10.1159/000337094.
- Fuhrer M, Durner J, Brunnler G, et al. HLA association is different in children and adults with severe acquired aplastic anemia. Pediatr Blood Cancer. 2007;48(2):186–191. DOI:10.1002/pbc.20785.
- Oguz FS, Yalman N, Diler AS, et al. HLA-DRB1*15 and pediatric aplastic anemia. Haematologica. 2002;87(7):772–774.
- Yin S, Ma W, Zhu W, et al. Immunogenetic association of HLA class I, II with aplastic anemia in Chinese population. Med J Chin People's Armed Police Forces. 1997;5:255–256.
- Camitta B. M, Store R, Thomas E. D. Aplastic anemia. Pathogenesis, diagnosis, treatment and prognosis. N Engl J Med. 1982;306:645–652. doi: 10.1056/NEJM198203183061105
- Li J, Jia G, Liu X. Advances in the pathogenesis of aplastic anemia. Chin J Lab Diagnosis. 2017;21(1):165–168.
- Aggarwal S, Pittenger MF. Human mesenchymal stem celts rood ulate allogeneic immune responses. Blood. 2005;i05(4):1815. doi: 10.1182/blood-2004-04-1559
- Zhao L, Xiao Y, Jin R. The progress of pathogenesis of aplastic anemia. Chin J Pract Pediatr. 2011;18(7):545–548.
- Liu C, Li Z, Sheng W, et al. Abnormalities of quantities and functions of natural killer cells in severe aplastic anemia. Immunol Invest. 2014;43(5):491–503. DOI:10.3109/08820139.2014.888448.
- Wang T, Mei SC, Fu R, et al. Expression of shelter in component POT1 is associated with decreased telomere length and immunity condition in humans with severe aplastic anemia. Res J Immunol. 2013;2014(2014):439530.
- Usman M, Adil SN, Moatter T, et al. Increased expression of HLA DR2 in acquired aplastic anemia and its impact on response to immunosuppressive therapy. J Pak Med Assoc. 2004;54(5):251–254.
- Saunthararajah Y, Nakamura R, Nam JM, et al. HLA-DR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood. 2002;100(5):1570–1574.
- Lu SY, Xiao LL, Luo M, et al. Relationship between polymorphism of HLA-A, -B, -DRB1 alleles and susceptibility of children to acquired aplastic anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20(1):120–124.