ABSTRACT
Objectives: To investigate the clinical and prognostic significance of absolute basophil count (ABC) in patients with primary myelofibrosis (PMF).
Methods: We retrospectively investigated 58 patients with PMF treated in our institution in the period from 2006 to 2017. ABC was obtained in addition to other hematological and clinical parameters. Patients were separated into high and low ABC groups using the Receiver operating characteristic curve analysis.
Results: ABC was higher in PMF patients than in healthy controls (P < 0.001). Patients with high ABC had higher white blood cells (P < 0.001), higher red cell distribution width (P = 0.035), higher lactate dehydrogenase (P < 0.001), more frequently had circulatory blasts (P < 0.001), constitutional symptoms (P = 0.030) and massive splenomegaly (P = 0.014). ABC was also positively correlated with absolute monocyte count (AMC) (P < 0.001) and other components of differential blood count. There was no difference in ABC regarding driver mutations or degree of bone marrow fibrosis. Univariately, high ABC was significantly associated with inferior overall survival (hazard ratio (HR) 4.79, P < 0.001). This effect remained statistically significant (HR 4.27, P = 0.009) in a multivariate Cox regression model adjusted for age, gender, Dynamic International Prognostic Scoring System (HR 2.6, P = 0.001) and AMC (HR 8.45, P = 0.002).
Discussion: High ABC reflects higher disease activity and stronger proliferative potential of disease. ABC and AMC independently predict survival and therefore seem to reflect different underlying pathophysiologic processes. Hence, both have a potential for improvement of current prognostic scores.
Conclusion: Basophils represent a part of malignant clone in PMF and are associated with unfavorable disease features and poor prognosis which is independent of currently established prognostic scoring system and monocytosis.
Introduction
Primary myelofibrosis (PMF) is a Philadelphia chromosome negative myeloproliferative neoplasm (Ph-MPN) [Citation1] originating from clonally transformed hematopoietic stem cell [Citation2]. Most patients carry a mutation in either Janus kinase 2 (JAK2), calreticulin (CALR) or myeloproliferative leukemia virus oncogene (MPL) genes [Citation3] resulting in activation of JAK-signal-transducer-and-activator-of-transcription signaling pathway which is central to disease pathogenesis. Disease is characterized by strong myeloproliferation, development of bone marrow fibrosis, egress of blasts into peripheral blood and development of extramedullary hematopoiesis, mostly in the liver and spleen. Pronounced inflammatory atmosphere present in PMF commonly results in debilitating constitutional symptoms and strongly reflects on decreased quality of life in these patients [Citation4].
PMF is characterized by more aggressive biological behavior and higher mortality in comparison to other Ph-MPNs [Citation5]. Risk of death can be stratified by several prognostic systems, the International Prognostic Scoring System [Citation6] and the Dynamic International Prognostic Scoring System (DIPSS) [Citation7] being most appropriate at the time of diagnosis and during the patient follow-up, respectively, if karyotype is unknown. Both scores account for patient's age, white blood cells (WBC), hemoglobin level, circulatory blasts and constitutional symptoms. Karyotype, platelet count and transfusion status are additionally included into the DIPSS-Plus prognostic model [Citation8].
Besides factors included into the currently established prognostic scores, a variety of other disease-associated parameters can contribute to better prognostic discrimination in PMF patients, such as lactate dehydrogenase (LDH) levels [Citation9], red cell distribution width (RDW) [Citation10] and monocytosis in the peripheral blood [Citation11]. Elevation in the number of circulatory basophils is a known feature of both chronic myelogenous leukemia (CML) and Ph-MPNs [Citation12–15]. In CML, basophilia is recognized as an important diagnostic and prognostic parameter and it is included into the variety of prognostic scores [Citation16–19]. However, there are limited insights into the possible role of basophils in Ph-MPNs [Citation20] and their role in the pathogenesis and prognosis of PMF patients is currently unknown.
In this study, we investigate the clinical and prognostic significance of absolute basophil count (ABC) in patients with PMF and show its powerful prognostic potential independent of both the DIPSS and elevated absolute monocyte count (AMC).
Patients and methods
A total of 58 patients with PMF with available data that were evaluated in our institution in the period from 2006 to 2017 and were fulfilling 2016 WHO criteria for PMF diagnosis [Citation1] were included into this study. A total of 49 (84.5%) patients were evaluated at the time of diagnosis and nine (15.5%) patients were evaluated during a follow-up period. All patients provided written informed consent for molecular analyses. The study was approved by the Institutional Review Board.
ABC was determined using automated cell counter and was recorded in addition to other hematological and clinical parameters (WBC, absolute neutrophil, lymphocyte, monocyte, and eosinophil counts, circulatory blasts, hemoglobin level, platelets, RDW, C reactive protein (CRP), LDH, age, gender, transfusional dependency, constitutional symptoms, blast phase disease, JAK2, CALR or MPL mutational status). The size of the spleen and liver were assessed by palpation. Bone marrow fibrosis was graded according to the current European consensus [Citation21]. Data regarding karyotype were unavailable for some studied patients and disease was staged according to the DIPSS, rather than the DIPSS-plus prognostic scoring system to avoid power limitations. ABC of PMF patients was compared to that of 30 age- and gender-matched healthy controls.
For mutation testing, DNA was isolated from full blood by the QIAamp DNA Blood Mini Kit (Qiagen, ID 51104). JAK2 V617F was assessed by the allele-specific polymerase chain reaction (PCR), as described previously [Citation22], CALR1 and MPL exon 10 mutations were screened by the high–resolution melting dye assays [Citation23,Citation24] and any sample sequence that deviated from normal was Sanger-sequenced.
The normality of data distribution was tested using the Kolmogorov–Smirnov test. Normally distributed numerical variables were presented as arithmetic mean ± standard deviation and non-normally distributed numerical variables were presented as median and interquartile range (IQR). ABC values were non-normally distributed. The T-test, the Mann–Whitney U test, the Kruskal–Wallis test, the χ2 (Chi squared) test, the Spearman rank correlation and the Jonckheere–Terpstra test for trend were used where appropriate. Survival analyses were performed using the methods of Kaplan and Meier, the Cox–Mantel version of the log-rank test [Citation25] and Cox regression analysis. Receiver operating characteristic (ROC) curve analysis using survival status as classification variable was performed for determining an optimal ABC cut-off value for survival analyses. P-values < 0.05 were considered statistically significant. Associations of potential prognostic factors with survival were screened for using the custom-made MS Excel workbook [Citation26]. Analyses were performed using the MedCalc Statistical Software version 17.6 (MedCalc Software BVBA, Ostend, Belgium).
Results
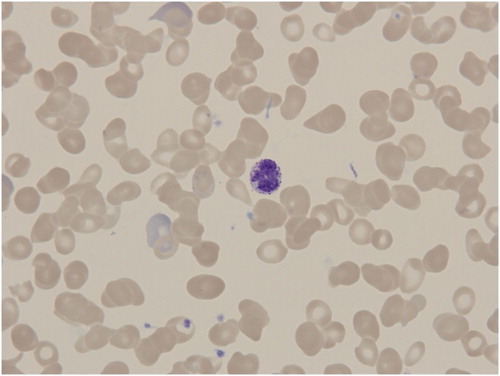
There were a total of 58 PMF patients, 37 (63.8%) of them were males. The mean age at the time of evaluation was 66.5 ± 9.4 years. Circulating basophil in a blood smear of one of our PMF patients is shown in .
Figure 1. Peripheral blood smear in PMF patient showing circulating basophile among characteristically deformed red blood cells (dacryocytes).
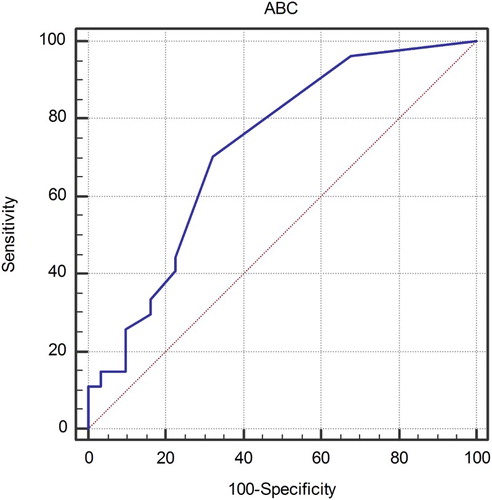
ABC was significantly higher in PMF patients than in healthy controls (median 0.15 × 109/L, IQR (0.1–0.3) vs. 0 × 109/L, IQR (0–0), P < 0.001) with 81% of PMF patients exceeding the upper limit of ABC reference range of 0.06 × 109/L used in our laboratory. Using the ROC curve analysis, optimal ABC cut-off value for the purpose of survival analyses was set at 0.1 × 109/L and patients were separated into ‘High ABC’ group (>0.1 × 109/L) and ‘Low ABC’ group (≤0.1 × 109/L). ROC curve is shown in . Patients’ characteristics stratified by ABC categories are shown in .
Table 1. Patients’ characteristics stratified in two groups: ABC >0.1 (high ABC) and ≤0.1 (low ABC).
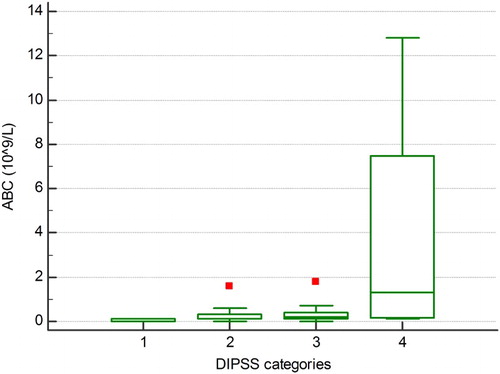
Patients with high ABC had higher WBC (P < 0.001), RDW (P = 0.035), LDH (P < 0.001), more frequently had circulatory blasts (P < 0.001), constitutional symptoms (P = 0.030) and massive splenomegaly (P = 0.014). We also observed that ABC as a continuous variable was correlated not only with WBC (P < 0.001) and percentage of circulatory blasts (P < 0.001) but also with the other components of differential blood count: absolute neutrophil count (P < 0.001), absolute lymphocyte count (P = 0.067), AMC (P < 0.001) and absolute eosinophil count (P < 0.001). We also observed a significant increasing trend in ABC over DIPSS categories (P = 0.002), as shown in . ABC was significantly higher in all DIPSS-risk groups than in controls. We observed no statistically significant relationship between ABC and degree of bone marrow fibrosis, hemoglobin, platelet count, CRP, age, gender, transfusional dependency, blast phase disease, JAK2, CALR or MPL mutational status.
Figure 3. There was a significant trend of increase in ABC over DIPSS categories, Jonckheere–Terpstra trend test, P = 0.002. 1 = low risk, 2 = intermediate-1 risk, 3 = intermediate-2 risk, 4 = high risk.
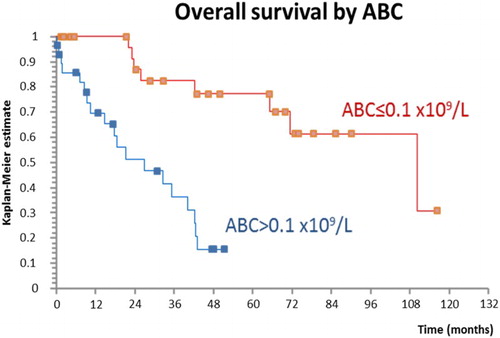
High ABC was significantly associated with inferior overall survival in univariate analysis (hazard ratio (HR) 4.79, P < 0.001) as shown in . The negative effect of high ABC on survival remained significant after adjustment for age, gender and the DIPSS in a multivariate Cox regression model: both high ABC (HR 5.26, P = 0.002) and the DIPSS (HR 2.95, P < 0.001) were able to predict survival independently of each other. In addition, we created a Cox regression model, including age, gender, high ABC, the DIPSS and AMC >1.4 (cut-off value was determined using the ROC curve analysis for maximization of effect of monocytes on survival): high ABC (HR 4.27, P = 0.009), high AMC (HR 8.45, P = 0.002) and the DIPSS (HR 2.6, P = 0.001) each have additional prognostic properties and were able to predict poor survival independently of each other.
Figure 4. ABC >0.1 was associated with inferior overall survival, log-rank test, HR 4.79, P < 0.001.
We further created a model comparing ABC, AMC and WBC since WBC by definition includes ABC and AMC and is a part of current prognostic scores: ABC (HR 5.62, P = 0.002), AMC (HR 6.45, P = 0.002) and WBC (HR 1.03, P = 0.024) were all independently associated with inferior survival showing that ABC and AMC provide additional prognostic information above WBC. Finally, we compared prognostic properties of ABC and AMC in a model including the DIPSS, age, gender, WBC, platelets < 100 × 109/L and transfusion status () where ABC (HR 4.04, P = 0.021), AMC (HR 6.18, P = 0.013) and the DIPSS (HR 2.38, P = 0.009) remained significantly associated with inferior survival.
Table 2. Overview of a Cox regression model showing that ABC >0.1, AMC >1.4 and the DIPSS have additional prognostic properties and are able to predict overall survival in PMF patients independently of each other.
Discussion
To the best of our knowledge, this is the first study to evaluate the clinical and prognostic significance of basophils in PMF and to show the powerful prognostic potential of elevated ABC that is independent of both currently established prognostic score (DIPSS) and monocytosis. Our results also confirm previous observations regarding the role of monocytosis in the prediction of adverse outcomes in PMF patients [Citation11,Citation27].
As mentioned previously, increased circulatory basophils are a known feature of both Philadelphia chromosome-positive and -negative MPNs [Citation12–15]. In contrast to CML, where bone marrow basophils are also known to be increased [Citation28,Citation29], bone marrow basophils in PMF, essential thrombocytosis (ET) and polycythemia rubra vera (PRV) do not seem to be elevated in comparison to normal controls [Citation28,Citation29]. We identified only one prior study assessing clinical features and basophil counts in Ph-MPNs, but it was not primarily focused on PMF patients, nor did it assess correlation with other disease-specific features besides JAK2 mutational status in PMF. In this study by Pieri et al. [Citation20], the primary focus was on PRV patients with aquagenic pruritus and only 22 PMF patients were included. Authors found increased ABC in patients with PMF in comparison to controls which is in line with our and earlier observation. They also observed that higher ABCs were associated with the JAK2 V617F mutation in PMF and ET which could not be confirmed in our study. It should be noted that we did observe higher ABC in JAK2-mutated patients, but this result did not reach statistical significance. Authors also noted that activated basophil counts in PMF and ET patients did not differ in comparison to controls (absolute basophil counts were elevated). This is in contrast to PRV, where absolute and activated basophil counts were increased and activated basophil count correlated with JAK2 V617F allele burden and symptoms of aquagenic pruritus. Authors did not investigate correlations of ABC with other clinical features in PMF. Therefore, ours is the first study to report the association of higher ABC with more advanced disease features and elaborate possible role in the pathogenesis and prognosis of disease.
In our cohort of PMF patients, higher ABC was associated with elevation in WBC, circulatory blasts and other components of differential blood count, as well. Therefore, higher ABC is associated with augmented hematopoiesis and reflects stronger proliferative potential of disease. Higher disease activity is also reflected in elevated LDH, massive splenomegaly and constitutional symptoms seen with elevated ABC. There were no significant differences in the distribution of driver mutations between high and low ABC groups. Basophils are circulating effector cells that harbor a large number of pro-inflammatory mediators inside metachromatic granules such as biogenic amines, leukotrienes and cytokines such as interleukin (IL)-4, IL-13 and granulocyte-macrophage colony-stimulating factor (GM-CSF) [Citation30–32]. An increased number of circulating basophils can thus contribute to high inflammatory atmosphere characteristic for disease, might promote atherosclerosis [Citation33] and potentiate development of constitutional symptoms as seen in our study.
Our most interesting observation is the prognostic potential of ABC. ABC correlated with different known negative prognostic factors in PMF such as the DIPSS score [Citation7] and related factors (WBC, circulatory blasts and constitutional symptoms), LDH [Citation9], RDW [Citation10] and AMC [Citation11]. In addition, ABC bears powerful prognostic properties itself. This can be demonstrated both in univariate and in multivariate analyses in which effect was independent of the DIPSS and elevated monocyte count. This is of particular interest, because it suggests that although ABC and AMC are positively correlated and both probably represent stronger proliferative potential of disease, they seem to reflect different underlying pathophysiologic processes. It should be noted that basophilia has been reported in hematologic malignancies harboring lesions of chromosome 9, e.g. CML (Philadelphia chromosome t(9;22)(q34;q11)) [Citation13,Citation28,Citation29] and acute myelogenous leukemia with t(6;9)(p23;q34) [Citation34–36]. Trisomy 9 has been recognized as a recurrent cytogenetic abnormality in patients with PMF, usually with favorable prognosis if occurring as a sole abnormality [Citation37–39], but can also be associated with additional cytogenetic lesions [Citation38]. JAK2 gene is located on chromosome 9p24.1 and patients with PRV and ET seem to have higher JAK2 allele burden if harboring +9 [Citation40]. We speculate that the increase in ABC seen in PMF patients could be mediated through lesions on chromosome 9 in which obviously a locus minoris resistentiae for disease exists. Also, since both ABC and AMC provide additional prognostic information independently of each other and established prognostic score, both ABC and AMC have a potential for improvement of current prognostic systems.
Limitations of our study are small number of patients, retrospective study design, single-center experience and inability to obtain karyotype of all studied patients. Therefore, no conclusion regarding association with chromosome 9 lesions is possible at the moment. However, this interesting phenomenon needs to be further investigated in new and larger PMF cohorts.
In conclusion, basophils represent a part of malignant clone in PMF and their elevation in peripheral blood is associated with unfavorable disease features and poor prognosis which is independent of the currently established prognostic scoring system and monocytosis. ABC is a simple and easily obtainable biomarker with powerful prognostic properties that can help in rapid recognition of patients with unfavorable prognosis.
Ethical approval
The study was approved by the Institutional Review Board.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Marko Lucijanic http://orcid.org/0000-0002-1372-2040
Ana Livun http://orcid.org/0000-0002-6758-1677
Jelena Lucijanic http://orcid.org/0000-0001-7566-8708
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391–2405. doi:10.1182/blood-2016-03-643544. PubMed PMID: 27069254. doi: 10.1182/blood-2016-03-643544
- Buschle M, Janssen JW, Drexler H, et al. Evidence for pluripotent stem cell origin of idiopathic myelofibrosis: clonal analysis of a case characterized by a N-ras gene mutation. Leukemia. 1988 Oct;2(10):658–660. PubMed PMID: 3050294.
- Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014 Jul;28(7):1472–1477. doi:10.1038/leu.2014.3. PubMed PMID: 24402162. doi: 10.1038/leu.2014.3
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients. Cancer. 2007 Jan 01;109(1):68–76. doi:10.1002/cncr.22365. PubMed PMID: 17123268. doi: 10.1002/cncr.22365
- Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012 Aug 20;30(24):2995–3001. doi:10.1200/JCO.2012.42.1925. PubMed PMID: 22802311; PubMed Central PMCID: PMC3417050. doi: 10.1200/JCO.2012.42.1925
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009 Mar 26;113(13):2895–2901. doi:10.1182/blood-2008-07-170449. PubMed PMID: 18988864. doi: 10.1182/blood-2008-07-170449
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010 Mar 4;115(9):1703–1708. doi:10.1182/blood-2009-09-245837. PubMed PMID: 20008785. doi: 10.1182/blood-2009-09-245837
- Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011 Feb 1;29(4):392–397. doi:10.1200/JCO.2010.32.2446. PubMed PMID: 21149668. doi: 10.1200/JCO.2010.32.2446
- Shah S, Mudireddy M, Barraco D, et al. Marked elevation of serum lactate dehydrogenase (LDH) in primary myelofibrosis: clinical and prognostic correlates. Blood. 2016;128(22):3113–3113.
- Lucijanic M, Pejsa V, Jaksic O, et al. The degree of anisocytosis predicts survival in patients with primary myelofibrosis. Acta Haematol. 2016;136(2):98–100. doi:10.1159/000445247. PubMed PMID: 27189016. doi: 10.1159/000445247
- Shah S, Mudireddy M, Lasho TL, et al. Monocytosis is a powerful and independent predictor of shortened overall and leukemia-free survival in primary myelofibrosis. Blood. 2016;128(22):4249–4249.
- Juhlin L. Basophil and eosinophil leukocytes in various internal disorders. Acta Med Scand. 1963;174(2):249–255. doi: 10.1111/j.0954-6820.1963.tb07918.x
- Spiers AS, Bain BJ, Turner JE. The peripheral blood in chronic granulocytic leukaemia. Study of 50 untreated Philadelphia-positive cases. Scand J Haematol. 1977 Jan;18(1):25–38. PubMed PMID: 265093. doi: 10.1111/j.1600-0609.1977.tb01474.x
- Gilbert HS. Myelofibrosis revisited: characterization and classification of myelofibrosis in the setting of myeloproliferative disease. Prog Clin Biol Res. 1984;154:3–17.
- Arnalich F, Lahoz C, Larrocha C, et al. Incidence and clinical significance of peripheral and bone marrow basophilia. J Med. 1987;18(5-6):293–303. PubMed PMID: 3505257.
- Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011 Jul 21;118(3):686–692. doi:10.1182/blood-2010-12-319038. PubMed PMID: 21536864. doi: 10.1182/blood-2010-12-319038
- Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing committee for the collaborative CML prognostic factors project group. J Natl Cancer Inst. 1998 Jun 03;90(11):850–858. PubMed PMID: 9625174. doi: 10.1093/jnci/90.11.850
- Braga GW, Chauffaille ML, Moncau JE, et al. Chronic myeloid leukemia (CML): prognostic factors and survival analysis. Sao Paulo Med J. 1996 Jan-Feb;114(1):1083–1090. PubMed PMID: 8984584. doi: 10.1590/S1516-31801996000100005
- Steegmann J, Odriozola J, Rodriguez-Salvanes F, et al. Stage, percentage of basophils at diagnosis, hematologic response within six months, cytogenetic response in the first year: the main prognostic variables affecting outcome in patients with chronic myeloid leukemia in chronic phase treated with interferon-alpha. Results of the CML89 trial of the Spanish Collaborative Group on Interferon-alpha2a and CML. Haematologica. 1999;84(11):978–987.
- Pieri L, Bogani C, Guglielmelli P, et al. The JAK2V617 mutation induces constitutive activation and agonist hypersensitivity in basophils from patients with polycythemia vera. Haematologica. 2009;94(11):1537–1545. doi:10.3324/haematol.2009.007047. PubMed PMID: PMC2770964. doi: 10.3324/haematol.2009.007047
- Thiele J, Kvasnicka HM, Facchetti F, et al. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005 Aug;90(8):1128–1132. PubMed PMID: 16079113.
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19-25;365(9464):1054–1061. doi:10.1016/S0140-6736(05)71142-9. PubMed PMID: 15781101. doi: 10.1016/S0140-6736(05)74230-6
- Bilbao-Sieyro C, Santana G, Moreno M, et al. High resolution melting analysis: a rapid and accurate method to detect CALR mutations. PloS one. 2014;9(7):e103511. doi:10.1371/journal.pone.0103511. PubMed PMID: 25068507; PubMed Central PMCID: PMC4113452. doi: 10.1371/journal.pone.0103511
- Pardanani A, Guglielmelli P, Lasho TL, et al. Primary myelofibrosis with or without mutant MPL: comparison of survival and clinical features involving 603 patients. Leukemia. 2011 Dec;25(12):1834–1839. doi:10.1038/leu.2011.161. PubMed PMID: 21691276. doi: 10.1038/leu.2011.161
- Lucijanic M, Skelin M, Lucijanic T. Survival analysis, more than meets the eye. Biochem Med. 2017 Feb 15;27(1):14–18. doi:10.11613/BM.2017.002. PubMed PMID: 28392721; PubMed Central PMCID: PMC5382852. doi: 10.11613/BM.2017.002
- Lucijanic M. Survival analysis in clinical practice: analyze your own data using an excel workbook. Croat Med J. 2016 Feb 29;57(1):77–79. PubMed PMID: 26935618. doi: 10.3325/cmj.2016.57.77
- Elliott MA, Verstovsek S, Dingli D, et al. Monocytosis is an adverse prognostic factor for survival in younger patients with primary myelofibrosis. Leuk Res. 2007;31(11):1503–1509. doi:10.1016/j.leukres.2006.12.025. PubMed PMID: 17397921. doi: 10.1016/j.leukres.2006.12.025
- Agis H, Krauth MT, Bohm A, et al. Identification of basogranulin (BB1) as a novel immunohistochemical marker of basophils in normal bone marrow and patients with myeloproliferative disorders. Am J Clin Pathol. 2006 Feb;125(2):273–281. doi:10.1309/M9FQ-MQGF-6616-7N2X. PubMed PMID: 16393678. doi: 10.1309/M9FQMQGF66167N2X
- Agis H, Krauth MT, Mosberger I, et al. Enumeration and immunohistochemical characterisation of bone marrow basophils in myeloproliferative disorders using the basophil specific monoclonal antibody 2D7. J Clin Pathol. 2006;59(4):396–402. doi:10.1136/jcp.2005.029215. PubMed PMID: 16461568; PubMed Central PMCID: PMC1860377. doi: 10.1136/jcp.2005.029215
- Schroeder JT, MacGlashan DW, Jr, Lichtenstein LM. Advances in immunology. Adv Immunol. 2001;77:93–122. PubMed PMID: 11293121. doi: 10.1016/S0065-2776(01)77015-0
- Stone KD, Prussin C, Metcalfe DD. Ige, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–S80. doi:10.1016/j.jaci.2009.11.017. PubMed PMID: PMC2847274. doi: 10.1016/j.jaci.2009.11.017
- Valent P, Bettelheim P. The human basophil. Crit Rev Oncol/Hematol. 1990;10:327–352. doi: 10.1016/1040-8428(90)90009-H
- Soylu K, Gulel O, Yucel H, et al. The effect of blood cell count on coronary flow in patients with coronary slow flow phenomenon. Pak J Med Sci. 2014 Sep-Oct;30(5):936–941. doi:10.12669/pjms.305.4935. PubMed PMID: PMC4163207.
- Chi Y, Lindgren V, Quigley S, et al. Acute myelogenous leukemia with t(6;9)(p23;q34) and marrow basophilia: an overview. Arch Pathol Lab Med. 2008 Nov;132(11):1835–1837. doi:10.1043/1543-2165-132.11.1835. PubMed PMID: 18976025.
- Pearson MG, Vardiman JW, Le Beau MM, et al. Increased numbers of marrow basophils may be associated with a t(6;9) in ANLL. Am J Hematol. 1985 Apr;18(4):393–403. PubMed PMID: 3976650. doi: 10.1002/ajh.2830180409
- Alsabeh R, Brynes RK, Slovak ML, et al. Acute myeloid leukemia with t(6;9) (p23;q34): association with myelodysplasia, basophilia, and initial CD34 negative immunophenotype. Am J Clin Pathol. 1997 Apr;107(4):430–437. PubMed PMID: 9124211. doi: 10.1093/ajcp/107.4.430
- Hussein K, Van Dyke DL, Tefferi A. Conventional cytogenetics in myelofibrosis: literature review and discussion. Eur J Haematol. 2009 May;82(5):329–338. doi:10.1111/j.1600-0609.2009.01224.x. PubMed PMID: 19141119. doi: 10.1111/j.1600-0609.2009.01224.x
- Tefferi A, Mesa RA, Schroeder G, et al. Cytogenetic findings and their clinical relevance in myelofibrosis with myeloid metaplasia. Br J Haematol. 2001 Jun;113(3):763–771. PubMed PMID: 11380468. doi: 10.1046/j.1365-2141.2001.02796.x
- Caramazza D, Begna KH, Gangat N, et al. Refined cytogenetic-risk categorization for overall and leukemia-free survival in primary myelofibrosis: a single center study of 433 patients. Leukemia. 2011 Jan;25(1):82–88. doi:10.1038/leu.2010.234. PubMed PMID: 20944670; PubMed Central PMCID: PMC3035978. doi: 10.1038/leu.2010.234
- Li M, Wen L, Cen J, et al. JAK2V617F allele burden in patients with myeloproliferative neoplasms carrying trisomy 9, and its relationship with clinical phenotypes. Int J Hematol. 2016 May;103(5):599–601. doi:10.1007/s12185-016-1986-2. PubMed PMID: 27084251. doi: 10.1007/s12185-016-1986-2