ABSTRACT
Objectives: Newer tyrosine kinase inhibitors (TKIs) (bosutinib, ponatinib) and allogeneic hematopoietic stem cell transplantation (allo-HSCT) can be utilized as a salvage therapy in patients with chronic myeloid leukemia (CML) who failed two lines (imatinib → nilotinib or imatinib → dasatinib) of TKI therapy. However, these TKIs are not available in many countries and not all patients can undergo allo-HSCT.
Methods: In this study, CML patients who received dasatinib or nilotinib as a third-line treatment were retrospectively evaluated.
Results: Out of 209 patients, third-line dasatinib/nilotinib was administered in 21. During the follow-up, 16 out of 21 patients gained and/or maintained an optimal response, and 4 patients died due to progression. Seventeen patients were alive at the time of the analysis, of which 13 were still on TKI, whereas 4 patients quit treatment.
Discussion: In patients failing two lines of TKI, dasatinib or nilotinib can be beneficial and safely administered as a third-line treatment especially in nations with restricted resources.
Introduction
Imatinib (Gleevec/Glivec; Novartis Pharmaceuticals) is a BCR-ABL1 tyrosine kinase inhibitor (TKI) that has dramatically improved the outcome of patients with chronic myeloid leukemia (CML). Although most of the patients with CML in chronic phase (CML-CP) do well under imatinib, approximately 40% of them quit receiving imatinib due to lack of efficacy and/or because of intolerance. In the imatinib arm of the International Randomized Study of Interferon and STI571 (IRIS) trial, 34% of patients were no longer on the study drug at 6 years, for reasons that included lack of efficacy (12%) and the occurrence of adverse events (AEs) (4%) [Citation1]. Nearly, 20% of the patients develop resistance (both primary and secondary) [Citation2], and BCR-ABL1 kinase domain (KD) mutations are detected in approximately 50% of patients with treatment failure and progression [Citation3]. Second-generation TKIs (2GTKIs) (dasatinib and nilotinib) are used in patients who have intolerance and resistance to imatinib [Citation4,Citation5], and it has been demonstrated that approximately 50% of patients failing to respond to previous treatments may respond to 2GTKIs [Citation6,Citation7]. Among imatinib-resistant patients with a KD mutation, in case of Y253H, E255K/V, or F359V/C/I mutations, dasatinib is probably more effective than nilotinib, whereas nilotinib should be chosen over dasatinib in patients harboring V299L, T315A, and F317L/V/I/C mutations [Citation8]. In addition to that, the choice between these two 2GTKIs is governed mostly by their potential toxicity profiles as well as the comorbidities of the patients [Citation9,Citation10]. Limited data are available, displaying the outcome of CML patients who failed two lines of TKI, and received 2GTKIs as a third-line treatment [Citation11–17]. The aim of this study is to report our single-center experience on CML patients who received dasatinib or nilotinib as a third-line treatment option, along with a literature review.
Patients and methods
Study population
We retrospectively evaluated 209 CML-CP patients who were diagnosed between 1999 and 2013. Patients’ demographics, Sokal risk scores, TKI treatment durations, and if any, treatments prior to imatinib (including interferon-alpha (IFN-α), cytarabine (Ara-C), and hydroxyurea (HU), but excluding short-term HU use for cytoreduction) and follow-up periods were noted from the patients’ files retrospectively. The efficacy of the treatment was evaluated using standard hematologic, molecular, and bone marrow cytogenetic assessments to determine rates of complete hematologic response (CHR), complete cytogenetic response (CCyR), and major molecular response (MMR).
Responses to TKIs and CML phases
Molecular response was classified based on BCR-ABL1 to control gene transcript ratios, expressed on the international scale, and MMR was defined as ratios ≤ 0.1%. Cytogenetic response based on bone marrow assessment was classified as complete (CCyR; 0% Ph+ cells), partial (PCyR; > 0–35% Ph+ cells), minor (>35–65% Ph+ cells), minimal (>65–95% Ph+ cells), and none (>95–100% Ph+ cells). Patient monitorization and response evaluation were both performed and defined according to European LeukemiaNet (ELN) recommendations [Citation3,Citation18], and in cases of resistance, mutational analysis was performed by Sanger sequencing as described before [Citation19]. The cumulative incidences of MMR and CCyR during the follow-up periods were calculated. CML phases were defined as described elsewhere [Citation3,Citation20].
Criterion used to choose 2GTKI
The choice of 2GTKI depended on the KD mutational status and comorbidities of the patient as recommended by ELN [Citation3,Citation8], and the disease phase at the time of the switch (i.e. in the advanced phase disease [accelerated phase (AP) and blast crisis (BC)], dasatinib was the treatment choice). Also in Turkey dasatinib was in the drug market before nilotinib became available, so during that period of time, dasatinib was the only option for a second-line TKI treatment. The starting dose for nilotinib was 2 × 400 mg daily, and dasatinib was administered with a dose of 100 mg/day in CML-CP patients and 140 mg/day for advanced disease.
Survival analysis
The patient group in which the 2GTKIs were used as a third-line treatment was further divided into two according to the reason for stopping the former TKI as ‘patients with failure (PF)’ or ‘patients with intolerance (PI)’. We calculated the duration of overall survival (OS) from the date of diagnosis until the time of death or last follow-up. The duration of event-free survival (EFS) was calculated from the onset of third-line TKI therapy until the date of any event (i.e. loss of any response, progression to advanced disease phases or switching to any other treatment). Kaplan–Meier method [Citation21] was used to determine the probability of OS or EFS for each patient group (PF and PI) and comparisons were done using the log-rank test. All tests were two-sided, and p < 0.05 was considered as statistically significant. All analyses were performed with the Statistical Package for the Social Sciences (SPSS) for Windows v.13.0 (SPSS Inc., Chicago, IL, USA).
Results
All patients in the initial cohort started TKI treatment with imatinib 400 mg/day. After a median duration of 87 months (range, 1–208 months), of the 209 patients, 2GTKIs were used in 65 (31%) (dasatinib in 45 and nilotinib in 20) – ‘1st switch’ (). In 58 of these 65 patients, 2GTKIs were administered due to resistance (both primary and secondary) to imatinib, in 6 patients, dasatinib or nilotinib was administered due to intolerance, and in one due to both intolerance and resistance (). During the follow-up, among those 59 imatinib-resistant cases, KD mutations were tested in 55 (93%). Thirty-seven patients (67%) remained wild-type (WT) throughout the entire follow-up, while 18 (33%) were tested as positive for at least one mutation. While choosing 2GTKI after imatinib resistance, KD mutational status of the patients, comorbidities and the disease phase at the time of the switch were all taken into consideration as stated in the methodology part.
Figure 1. The consort diagram showing the study cohort and treatment outcomes (2G, second generation; BC, blast crisis; CCyR, complete cytogenetic response; CML-CP, chronic myeloid leukemia in chronic phase; DAS, dasatinib; IM, imatinib; MMR, major molecular response; NIL, nilotinib; TKI, tyrosine kinase inhibitor). *One patient in each group died due to non-CML related causes while they were in MMR, the others deceased due to disease progression.
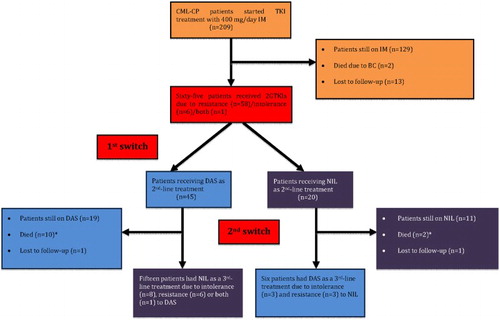
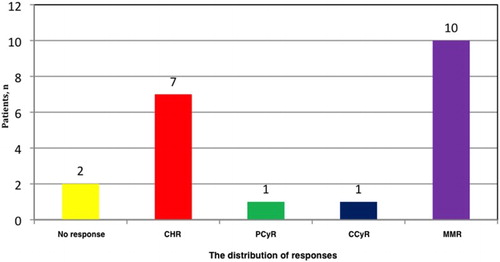
After a median duration of 24.5 months (range, 1–81 months) under second-line TKI therapy, 21 patients were switched to third-line 2GTKI treatment due to resistance/intolerance – ‘2nd switch’ (). demonstrates the clinical features and outcomes of these 21 patients who received 2GTKIs as a third-line therapy. There were 13 males, and the median age was 50 years (range, 20–67 years). Sokal risk score was calculated among 20 patients because 1 patient (patient #8) had splenectomy prior to the diagnosis of CML, and the percentages of low, intermediate, and high Sokal risk scores were 60%, 25%, and 15%, respectively. Thirteen patients (62%) received treatments prior to imatinib, and HU alone was administered in 7 and in combination with IFN-α in 3 cases. IFN-α+ARA-C was the treatment of choice in two patients whereas HU was added to this combination in one ().
Table 1. Baseline characteristics and treatment outcomes of patients who received third-line 2GTKIs.
Among the patients receiving third-line TKI treatment, after a median first-line TKI therapy with imatinib of 40 months (range, 2–123 months), 19 patients were switched to 2GTKIs due to resistance, whereas intolerance was the reason for stopping imatinib in 2 (myalgia in patient #13 and skin rash in patient #16) (). Best responses under imatinib were PCyR in 10 patients, MMR in 7 cases, CCyR in 3 patients, and CHR in 1. In patients who received 2GTKIs due to resistance, 5 harbored KD mutations under imatinib, and M244V was detected in 2 (patients #9 and #17), and E255K (patient #1), G250E (patient #15) and Y253H (patients # 19) were observed in 1. On the other hand, 14 patients were WT at the time of imatinib resistance ().
2GTKIs were administered as a second-line treatment with a median duration of 24 months (range, 1–56 months). Response evaluation was available in 19 patients, and best responses under second-line TKI therapy were MMR in 9 patients, PCyR in 8, CCyR and CHR in 1 patient each. Prior to third-line TKI therapy, 17 patients were WT and 2 (E255K in patient #1 and F317L in patient #18) had KD mutations (). In patient #1, the mutational analysis was sent and nilotinib was started thereafter, but after receiving the E255K result within a month, the TKI treatment was changed to dasatinib.
Nilotinib was administered in 15 patients after imatinib/dasatinib failure and 6 patients received dasatinib after imatinib/nilotinib failure (), and resistance to the former 2GTKI was the reason for switching in 9 patients, 11 patients, were switched to the subsequent treatment due to hematologic and/or non-hematologic AEs, and both failure and intolerance were observed in one case (patient #11) ().
With a median of 12 months (range, 1–42 months) of third-line TKI treatment, there were 16 out of 21 patients (76%) who gained and/or maintained an optimal response at given time points. The distribution of best responses achieved under third-line TKI therapy was displayed in . Among patients who were switched to third-line TKI treatment because of failure (PF Group; n = 9), eight had an optimal response, and among the patients who were switched to third-line TKI due to intolerance (PI Group; n = 11), an optimal response was achieved/maintained in nine.
During the follow-up, three patients (patients #2, #11, and #18) progressed to BC and died, and one (patient #8) deceased following allogeneic hematopoietic stem cell transplantation (allo-HSCT) due to disease progression. Seventeen patients were alive at the time of the analysis, of which 13 were still on TKI therapy, whereas 4 patients quit treatment, 3 (patients #4, #12, and #16) due to intolerance, and 1 (patient #14) because of both failure and intolerance after a median entire follow-up duration of 100 months (range, 45–208 months) ().
In patients where the reason for discontinuation of the third-line TKI treatment was intolerance, the duration for the current TKI therapy was less than 6 months, and 1 patient (patient #16) had MMR while 2 (patients #4, and #12) had CHR (). Among the 13 patients who were still on TKI, 9 of them (patients #1, #6, #7, #9, #10, #13, #15, #17, and #20) had MMR, and 1 (patient #19) had CCyR (this patient was on third-line dasatinib for only 7 months at the time of the evaluation). Among four patients who did not achieve a CCyR and were available for evaluation at the time of the analysis (i.e. received the third-line TKI therapy ≥ 6 months), best responses were PCyR in one patient (patient #2), and CHR in three (patients #3, #5, and #14) ().
Although EFS was superior in PI Group than in PF Group, the difference was not significant (p = 0.207) ((A)). OS was similar in both patient groups (p = 0.134) ((B)).
Figure 3. The event-free (A) and overall (B) survivals of patients when cases were divided into two according to the reason of switching to third-line TKI therapy as patients with failure (PF; n = 9) and intolerance (PI; n = 11) [OS was calculated from the date of diagnosis until the time of death or last follow-up. EFS was calculated from the onset of third-line TKI therapy until the date of any event defined].
![Figure 3. The event-free (A) and overall (B) survivals of patients when cases were divided into two according to the reason of switching to third-line TKI therapy as patients with failure (PF; n = 9) and intolerance (PI; n = 11) [OS was calculated from the date of diagnosis until the time of death or last follow-up. EFS was calculated from the onset of third-line TKI therapy until the date of any event defined].](/cms/asset/48cab256-6b3c-43e0-8e83-cd3c0953d7da/yhem_a_1385193_f0003_c.jpg)
Discussion
Although imatinib is most probably one of the most successful targeted therapy ever developed in cancer, approximately 40% of patients with CML-CP quit receiving imatinib due to failure and/or intolerance. Among our patient cohort, after a median duration of imatinib exposure of approximately 5 years, the cumulative CCyR and MMR rates were 87% and 81%, respectively, and these results were compatible with the 5-year follow-up data of the IRIS trial, which demonstrated a cumulative CCyR rate of 87% by 60 months [Citation22]. From the 209 patients who received imatinib as an upfront TKI treatment, 65 (31%) were switched to 2GTKIs due to failure or intolerance.
In case of imatinib resistance, while choosing a 2GTKI over another, BCR-ABL1 KD mutation status should be considered [Citation8], and among our patient cohort, 33% of the cases harbored a KD mutation in whom sensitive TKIs were administered for each defined mutation ().
The administration of 2GTKIs (dasatinib or nilotinib) has been shown to rescue nearly 50% of patients with CML [Citation6,Citation7]; however, the remaining cases need further treatment approaches. If these patients have no suitable donor and/or cannot undergo allo-HSCT, sequential treatment with other TKIs is a reasonable option. Although bosutinib and ponatinib, the newer TKIs, which can be utilized in patients with resistance and/or intolerance to imatinib and other 2GTKIs [Citation23,Citation24], these TKIs are not available in many countries in which, CML patients who failed two prior lines of TKI treatment are treated with either dasatinib or nilotinib as a third-line TKI therapy. Up to date, there is limited data accumulated in the literature regarding third-line TKI treatment with 2GTKIs (dasatinib or nilotinib) in patients with CML, which are displayed together with the present study in .
Table 2. The summary of studies displaying third-line 2GTKI treatment with dasatinib or nilotinib in the literature.
There were seven studies evaluating the use of 2GTKIs in the third-line setting [Citation11–17], of which in four of them, patients received both dasatinib and nilotinib as a third-line therapy [Citation12,Citation13,Citation15,Citation16], whereas in two [Citation11,Citation14], only one 2GTKI (dasatinib and nilotinib, respectively) was administered (). In one study, patients received bosutinib in addition to dasatinib and nilotinib as a third-line therapy [Citation17]. In our study, we included patients who only received dasatinib and nilotinib as a third-line treatment option.
Quintas-Cardama and colleagues [Citation11] reported 23 patients who received dasatinib as a third-line TKI therapy following imatinib and nilotinib treatment, and in all patients the reason for switching to dasatinib was failure. Their cohort consisted of patients mainly with advanced disease at diagnosis (10 patients with AP and 9 with BC). After a median follow-up of 34 weeks (range, 4–55 weeks), any response occurred in 13 (57%) patients of which 6 patients achieved CCyR and 4 had CHR as a best response [Citation11]. All our patients were in CP at the time of diagnosis, and six of them had dasatinib as third-line therapy (). Three out of these six patients were resistant to second-line nilotinib, and in those three cases MMR and CCyR were gained in one patient each, and CHR was achieved in one (). In the remaining three patients who quit second-line nilotinib due to intolerance, one had MMR and two achieved CHR as best responses under dasatinib.
Garg et al. [Citation12] reported a cohort of 48 CML patients who received third-line 2GTKI treatment (34 dasatinib and 14 nilotinib) after two lines of TKI treatment. Both failure and intolerance to prior TKIs were the reasons for switching to third-line therapy. After a median follow-up of 16 months (range, 3–34 months), 13 patients were still on therapy, and since the start of the third-line TKI, 25 patients (31%) were still alive, including 15 treated with dasatinib and 10 with nilotinib [Citation12]. The outcome of the patients varied by stage of the disease at the initiation of the third-line TKI and the authors suggested that third-line 2GTKI treatment may induce responses in some patients, but these are usually not durable except in occasional patients with CML-CP [Citation12]. Among our patient cohort, after a median of 12 months, which is relatively shorter than that of the study by Garg and colleagues, 17 (81%) of our patients were alive and 13 (62%) were still on TKI therapy.
Ibrahim and coworkers [Citation13] evaluated the efficacy of third-line TKI treatment with either dasatinib or nilotinib in 26 patients with CML-CP who failed 2 previous lines of TKI therapy. During the follow-up, 13 (50%), 9 (35%), and 5 (19%) patients achieved MCyR, CCyR, and MMR, respectively. Among their patient cohort, they showed that achieving any degree of cytogenetic response under first-line imatinib and/or during second-line TKI therapy were associated with a higher probability of gaining cytogenetic response with third-line setting [Citation13]. Also in our patient cohort, patients achieving cytogenetic/molecular responses were more likely to improve and/or maintain these responses (). Ibrahim et al. [Citation13] also showed that in their patient group, there were 17 cases who discontinued imatinib or second-line therapy due to grade III-IV non-hematologic toxicities, and among these patients, the probability of responding to the third-line therapy were similar to that of the resistant patients. This was also observed in our patients, and the percentages of optimal responders were comparable between resistant and intolerant cases (8/9; 89% and 9/11; 82%, respectively). Patients with cytogenetic resistance to the second-line therapy had a significantly worse EFS and OS in the cohort of İbrahim and colleagues [Citation13], whereas we did not observe such difference among our patients, which was most probably due to the different median durations of third-line TKI therapy in these two studies (21.5 months vs. 12 months).
In the study of the Giles et al. [Citation14], the authors evaluated 60 patients with CML (39 in CP and 21 in AP) who received third-line nilotinib. The percentages of patients who discontinued second-line dasatinib due to intolerance were 67% and 33% for patients in CP and AP, respectively. In addition to that, 80% of the cases did not achieve MCyR during dasatinib therapy with a median duration of more than 6 months [Citation14]. In our patient cohort, we had 15 cases with CML-CP who were exposure to third-line nilotinib, after a median duration of 25 months (range, 11–56 months) of dasatinib treatment (). Dasatinib was discontinued due to hematologic and non-hematologic AEs in eight patients (53%), failure was the reason for therapy cessation in six (40%), and one patient (7%) had both intolerance and failure. The main toxicity resulting in treatment discontinuation was pulmonary complications (), similar to what has been observed in the cohort of Giles and colleagues [Citation14]. Although myelosuppression under prior dasatinib therapy was one of the main causes resulting in switching to third-line nilotinib in their cohort [Citation14], we only had one case who experienced severe thrombocytopenia (also had PE) under dasatinib. Since we did not have any cases with advanced disease which was different than the study of Giles et al. [Citation14], this can be a reasonable explanation of the low frequency of grade III-IV hematologic AEs observed among our patient cohort under dasatinib.
In a multicenter Italian study consisting of 82 patients with CML-CP receiving third-line dasatinib (n = 34)/nilotinib (n = 48), 50% of the patients (n = 41) quit second-line TKI therapy due to failure, in 38 patients (46%) toxicities of prior 2GTKIs were the cause for treatment switch, and both failure and intolerance in 3 (4%) [Citation15]. These percentages were similar among our patient cohort, where the reasons for switching to third-line TKI therapy were intolerance, failure, and both in 52%, 43%, and 5% of the patients, respectively. Any cytogenetic response was achieved in 32 of the patients, and MMR was gained in 13, whereas disease progression occurred in 12 patients [Citation15]. In our cohort, 76% of the cases achieved and/or maintained an optimal response, whereas four patients died due to disease progression.
Ribeiro and colleagues evaluated 25 patients with CML who received third-line nilotinib/dasatinib therapy [Citation16]. Nine patients were switched to dasatinib, and 16 patients were switched to nilotinib as a third-line therapy. Of the CP patients (n = 18), 89% achieved CHR, 13% achieved a CCyR and 24% achieved MMR. Patients with advanced disease had inferior survival than patients with CML-CP. All of our patients were in CP, and the rates of best responses under third-line TKI therapy were 33%, 5%, 5%, and 48% for CHR, PCyR, CCyR, and MMR, respectively.
In a cohort of 53 patients with CML-CP, the authors evaluated the efficacy and safety of third-line therapy with 2GTKIs (dasatinib, nilotinib, and bosutinib) [Citation17]. Approximately 20% of the patients achieved CCyR during the follow-up, and progression to advanced disease was observed in 15% of the cases, which was 19% in our patient cohort.
In conclusion, although most of the patients with CML-CP achieve and maintain optimal responses under imatinib, some patients need salvage treatments. Among this patient group, second-line therapy with 2GTKIs can be administered with successful outcomes; however, some patients still need further treatment modalities due to failure and/or intolerance. Allo-HSCT is always a reasonable option, but it can only be performed in suitable patients (e.g. in patients with CP and good performance status, and with a suitable donor). Newer TKIs (bosutinib, ponatinib) can be utilized as a salvage therapy; however, these TKIs are not available in many countries. In patients failing two lines of TKI (imatinib → nilotinib or imatinib → dasatinib), dasatinib or nilotinib can be beneficial and safely administered as a third-line treatment especially in nations with restricted resources.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ahmet Emre Eskazan http://orcid.org/0000-0001-9568-0894
References
- Hochhaus A, O’Brien SG, Guilhot F, et al. Six year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. doi: 10.1038/leu.2009.38
- O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure or chronic myeloid leukemia. Blood. 2007;110(7):2242–2249. doi: 10.1182/blood-2007-03-066936
- Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569
- Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303–2309. doi: 10.1182/blood-2006-09-047266
- Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–3546. doi: 10.1182/blood-2007-03-080689
- Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. New Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229
- Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. New Engl J Med. 2006;354(24):2542–2551. doi: 10.1056/NEJMoa055104
- Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208–1215. doi: 10.1182/blood-2010-12-326405
- Carneiro BA, Kaplan JB, Giles FJ. Tyrosine kinase inhibitor therapy in chronic myeloid leukemia: update on key adverse events. Expert Rev Hematol. 2015;8(4):457–479. doi: 10.1586/17474086.2015.1041910
- Gugliotta G, Castagnetti F, Fogli M, et al. Impact of comorbidities on the treatment of chronic myeloid leukemia with tyrosine-kinase inhibitors. Expert Rev Hematol. 2013;6(5):563–574. doi: 10.1586/17474086.2013.837279
- Quintas-Cardama A, Kantarjian H, Jones D, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109(2):497–499. doi: 10.1182/blood-2006-07-035493
- Garg RJ, Kantarjian H, O’Brien S, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-up. Blood. 2009;114(20):4361–4368. doi: 10.1182/blood-2009-05-221531
- Ibrahim AR, Paliompeis C, Bua M, et al. Efficacy of tyrosine kinase inhibitors (TKIs) as third-line therapy in patients with chronic myeloid leukemia in chronic phase who have failed 2 prior lines of TKI therapy. Blood. 2010;116(25):5497–5500. doi: 10.1182/blood-2010-06-291922
- Giles FJ, Abruzzese E, Rosti G, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010;24(7):1299–1301. doi: 10.1038/leu.2010.110
- Russo Rossi A, Breccia M, Abruzzese E, et al. Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013;98(3):399–403. doi: 10.3324/haematol.2012.064337
- Ribeiro BF, Miranda EC, de Albuquerque DM, et al. Treatment with dasatinib or nilotinib in chronic myeloid leukemia patients who failed to respond to two previously administered tyrosine kinase inhibitors--a single center experience. Clinics (Sao Paulo). 2015;70(8):550–555. doi: 10.6061/clinics/2015(08)04
- Lomaia E, Zaritskey A, Shuvaev V, et al. Efficacy of tyrosine kinase inhibitors in third line therapy in chronic phase chronic myeloid leukemia. Blood. 2015;126(23):4051. [abstract]
- Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779
- Erbilgin Y, Catal S, Eskazan AE, et al. ABL gene kinase domain mutation scanning by denaturing high performance liquid chromatography sequencing method. Turk J Hematol. 2011;28(2):97–102. doi: 10.5152/tjh.2011.24
- Vardiman JW, Melo JV, Baccarani M, et al. Chronic myelogenous leukemia BCR-ABL1 positive. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO classification of tumors of hematopoietic and lymphoid tissues. Lyon: IARC; 2008. p. 32–37.
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452
- Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867
- Cortes JE, Kantarjian HM, Brümmendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17):4567–4576. doi: 10.1182/blood-2011-05-355594
- Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075–2088. doi: 10.1056/NEJMoa1205127