ABSTRACT
Objectives: Estrogen receptor beta (ERβ)-selective agonists inhibited B cell lymphoma growth in animal models. However, a recent study found that higher ERβ expression in tissue from diffuse large B cell lymphoma (DLBCL) patients indicated a poorer survival. This study aimed to determine the ERβ expression in DLBCL tissue using immunohistochemistry and correlate with clinical outcomes.
Methods: Diagnostic tissues from newly diagnosed adult DLBCL patients treated with Rituximab-Cyclophosphamide/Doxorubicin/Vincristine/Prednisolone were counted for ERβ1-expressing cells. Nodal lymphoma (N = 41) was analyzed separately from extra-nodal DLBCL (N = 31).
Results: On immunohistochemistry, ERβ1 was expressed in 73.6% of cases with the median expressing cells of 20%. For nodal lymphoma, high ERβ expression (≥25%) was associated with poorer event free survival (EFS) independent of the international prognostic index with the adjusted hazard ratio (HR) of 2.49 (95% Confidence interval (CI) 1.03–6.00, P = 0.042). On the contrary, high ERβ expression (≥25%) was associated with superior outcomes in extra-nodal DLBCL with the adjusted HR of 0.25 (95% CI 0.09–0.75, P = 0.013) for EFS and adjusted HR of 0.29 (95% CI 0.10–0.85, P = 0.024) for overall survival in multivariate analyses.
Conclusion: ERβ1 protein expression represented opposite prognostic factors in nodal vs. extra-nodal DLBCL.
Introduction
The incidence of B-cell lymphoid neoplasm, including chronic lymphocytic leukemia (CLL), Burkitt lymphoma, mantle cell lymphoma and diffuse large B cell lymphoma (DLBCL), is higher in male than in female population across all ethnic groups [Citation1]. Furthermore, previous studies suggest that exposure to estrogen during pregnancy, in the form of oral contraception or post-menopausal hormonal replacement is associated with lower risks for developing aggressive lymphoma [Citation2,Citation3]. Therefore, estrogen may play an inhibitory role in the pathogenesis of B-cell non-Hodgkin lymphoma. Nevertheless, some studies showed weak or no association between hormonal exposure and DLBCL incidences [Citation4–6]. There are several limitations of epidemiological investigations, such as difficulties in defining the exposure, variety in hormonal forms, as well as doses and/or durations, heterogeneity in lymphoma subtypes and unknown confounders associated with hormonal uses [Citation7]. Direct experimental research studies are required to draw definite conclusions.
Cellular actions of estrogen are mediated through estrogen receptors (ERs). There are two forms of nuclear ERs that are ERα and ERβ, as well as a cell surface-associated G protein-coupled ER or GPR30 [Citation8]. ERβ is present in normal B lymphoid cells in large quantity, while ERα is present in other tissues, such as reproductive organs [Citation9]. Experimental studies have shown that ERα confers growth and proliferative signals, while ERβ mediates anti-proliferative and pro-apoptotic effects on lymphoid cells [Citation9]. Selective ERβ receptor agonists, such as diarylpropionitrile and KB9520, have been developed [Citation10,Citation11]. These compounds have therapeutic potentials as the growth modulators of lymphoid malignancies.
Interestingly, Yakimchuk et al. reported that ERβ receptor was expressed in various kinds of B cell malignancies, including Burkitt lymphoma, mantle cell lymphoma, CLL and multiple myeloma [Citation12,Citation13]. Furthermore, they showed that the specific ERβ agonists could inhibit the growth of B cell lymphoma both in vitro and in a mouse xenograft model [Citation12]. The effects of ERβ agonists were more potent than those of the endogenous estradiol [Citation12], suggesting their promising role as a new targeted therapy for lymphoma. Consistent with these results, inhibition of estrogen production from androgenic substrates using an aromatase inhibitor enhances lymphoma growth in a mouse model [Citation14]. Notably, the activation of ERβ in lymphoma cells themselves, not in the microenvironment, inhibited angiogenesis and tumor dissemination, possibly through a decrease in vascular endothelial growth factor [Citation15,Citation16].
A recent study by the Hasni et al. found that selective ERβ inhibited DLBCL tumor cell lines in a xenograft mouse model [Citation17]. ERβ was detectable by immunohistochemistry in 89% of DLBCL tissues from clinical samples. Higher expression of ERβ1 proteins in DLBCL tissues of patients conferred a better prognosis in cases that were treated by Cyclophosphamide/Doxorubicin/Vincristine/Prednisolone (CHOP) chemotherapy. However, higher ERβ1expression was surprisingly associated with shorter survival in patients treated by CHOP plus rituximab (R), an anti-CD20 monoclonal antibody [Citation17]. Therefore, the effects of ERβ in patients may be more complicated than in animal models. An opposite results with concomitant immunotherapy may suggest an ERβ influence on the tumor–immune cell interactions in addition to the direct effects on lymphoma cell growth. In the animal xenograft models, cancer cell lines were injected into subcutaneous tissues of immune-deficient mice. Therefore, the tumor microenvironments are likely to be different in human DLBCL, especially for the nodal lymphoma where abundant immune cells are present.
As DLBCL is the most common type of lymphoma, this research aims to study the ERβ expression in Asians and correlate with disease prognosis. Additionally, the cut-off ERβ levels, which can predict outcomes, are determined. This work may yield a new prognostic biomarker and may give deeper insights in the roles of ERβ in DLBCL pathobiology.
Material and methods
Patients
This was an observational study including adult patients, aged over 18 years old, who were first diagnosed as DLBCL from biopsy according to the criteria of the WHO 2008 at King Chulalongkorn Memorial Hospital. Histopathology and immunohistochemistry of all specimens were reviewed by the experienced hematopathologist (T.A.). Patients were first diagnosed from 2008 to 2014 and treated with standard Rituximab-Cyclophosphamide/Doxorubicin/Vincristine/Prednisolone (R-CHOP) chemotherapy. In addition, there must have been remaining tissues available for this study.
The exclusion criteria were transformed lymphoma, HIV-associated lymphoma, concomitance with other types of cancer or previous treatments with chemotherapy.
Nodal and primary extra-nodal DLBCL were analyzed separately, because extra-nodal lymphoma may be more similar to the animal xenograft model, in which host immune cells are scarcer. Extra-nodal DLBCL was defined by the absence of apparent nodal disease. Nodal DLBCL patients had been followed up for at least 3 years, or death before 3 years. The minimal follow-up time for extra-nodal DLBCL was at least 2 years except for deaths or relapses before 2 years.
Method
All patient medical records were searched by ICD code of the disease (C833) and reviewed for the inclusion and exclusion criteria. Subsequently, the remaining paraffin-embedded lymph node tissues at initial diagnosis of the eligible patients were collected for immunohistochemical staining for ERβ1.
For immunohistochemistry, the tissue was sliced at 2 μm thick, de-paraffinized and antigen-retrieved using the target retrieval solution at high pH (PT LINK Module, DAKO). The primary antibody is the mouse monoclonal antibody to estrogen receptor beta (ERβ1) clone PPG 5/10 at 1: 40 dilution (Gene Tex). This clone was previously shown to be specific for ERβ1 isoform [Citation17]. The staining was performed using Autostain Link 48 (DAKO). The positive control was the ovarian tissue that expressed ER beta in granulosa cells. The negative control was a section without primary antibody.
Immunohistochemistry scoring was performed by the hematopathologist (T.A.) without the knowledge of clinical information. Positivity for ERβ was enumerated as the percentages of positively stained tumor cells. Because ERβ was expressed primarily in nuclei, only nuclear staining was enumerated. ERβ expression of over 1% of tumor cells was categorized as positive.
Cut-off point determination
The cut-off percentages of ERβ expression that could predict good responses were determined by the highest hazard ratio (HR) that was statistically significant. Subsequently, the expressing levels (High vs. low) were then correlated with the event free survival (EFS) in both univariate and multivariate analyses.
The definition of good response was the patients who attained complete remission (CR) after R-CHOP for 6–8 cycles and the disease did not recur until the end of follow-up. The follow-up end point was the date of last contact or death before December 2016. Poor response group included the patients who had disease progression or refractory disease or relapse or death with active disease after treatment.
EFS was measured from the date of the last treatment with R-CHOP to the date of relapse, disease progression, death or last contact.
Data analysis
Data were analyzed by the SPSS (version 20.0) program. The cases were categorized into primary nodal and primary extra-nodal DLBCL. The numerical data were shown as means ± standard deviations or medians with interquartile range (IQR) as appropriate. The comparisons of continuous and qualitative variables used t-tests, Mann–Whitney U-test or Chi-square as appropriate. EFS was determined by Kaplan–Meier method and association between factors and outcome were evaluated by the Cox regression model. The P values of less than 0.05 were considered statistical significance.
Results
Patient characteristics
From 2007 to 2016, 102 adult patients were first diagnosed as DLBCL and treated with the standard R-CHOP chemotherapy in King Chulalongkorn Memorial Hospital. After reviewing the patient data, 30 cases were excluded due to loss to follow-up (14), inadequate tissue sample (9), concomitance with other cancer (3), early deaths from severe infections after only 1 course of chemotherapy (2) and large cell transformation from low-grade lymphoma (2).
There were 72 cases included in the study. The nodal (N = 41) and primary extra-nodal (N = 31) subgroups were pre-specified to be separately analyzed. The mean age of the patients was 59.26 ± 13.21 (range was 18–85) years and 67.7% of them were male. Only six (8.3%) patients were premenopausal women, as defined by the age of equal to or younger than 48 years, which is the average menopausal age in Thailand.
The sites of extra-nodal DLBCL are gastrointestinal tract (9), upper airway (6), tonsils (6), hepatosplenic lymphoma without lymphadenopathy (2), soft tissue (2), thyroid (2), breast (1), ovary (1), kidney (1), skin (1) and bone marrow (1). The baseline characteristics of the patients from both groups are shown in .
Table 1. Baseline characteristics, ERβ expression and results of treatment of nodal vs. extra-nodal DLBCL.
All patients received complete treatment as plan. After R-CHOP treatment, 44 (61.1%) had CR. Cases with low or low-intermediate International prognostic index (IPI) or age-adjusted (aa) IPI were more likely to become CR with the odds ratio of 4.2, 95% confidence interval (CI) 1.2–14 (P = 0.022). Two more patients finally reached CR after following radiotherapy. Twelve (27.3%) of patients who achieved CR had relapses.
The expression of ERβ by immunohistochemistry
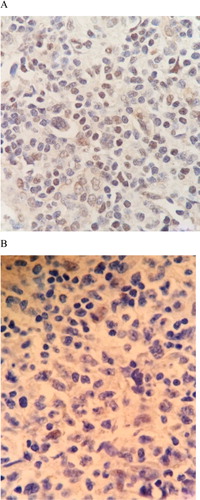
The majority of DLBCL tissue (73.6%) expressed variable degrees of ERβ protein by immunohistochemistry. The positive cells ranged from 0 to 100% with the median expression of 20% (IQR 0–80%). Representative examples are shown in . There was no statistical difference in ERβ1 protein between nodal and extra-nodal DLBCL, as shown in . Moreover, the expression of ERβ showed no correlation with gender or menopausal statuses. DLBCL tissue (N = 10) did not express ERα protein (data not shown).
ERβ expression and outcomes in the nodal lymphoma group
The mean ERβ expression in the good outcome group was 21.1 ± 29.9% (Median 5%, IQR 40%), while the mean expression in the poor outcome group was 40.5 ± 36.5% (Median 35%, IQR 70%). The difference between these two groups was significant (P = 0.037) by Mann–Whitney U-test.
The expression of 25% or more was associated with poor outcomes with the highest of HR that was statistical significant. The comparison of the baseline characteristics between low and high expression groups is shown in . There was no statistical difference in age, sex, performance status, advanced stage of diseases, elevated lactate dehydrogenase enzymes (LDH), the proportion of high–intermediate to high IPI risk or aaIPI, CR rate after R-CHOP and the proportion of non-germinal center B-cell (non-GCB) subtype of DLBCL as determined by immunohistochemistry using Hans criteria.
Table 2. The characteristics of nodal DLBCL patients (N = 41) divided by the expression of ERβ into low expression (<25%) and high expression (≥25%)
With the median follow-up time of 41 months, high (≥25%) ERβ expression was associated with shorter EFS with the HR of 2.53 (95% CI 1.07–5.99, P = 0.035) as shown in (A). High ERβ expression also showed a trend toward a poorer overall survival (OS), as in (B). As expected, high IPI was also associated with poorer EFS with the HR of 3.43 (95% CI 1.12–10.46, P = 0.03). In a multivariate analysis, both ERβ expression and IPI were independently associated with poor EFS (), but there was no statistical significance for OS.
Figure 2. The event free survivals (EFS) of nodal DLBCL. (A) High ERβ expression is associated with poor EFS in nodal DLBCL. (B) High ERβ expression and OS in nodal DLBCL.
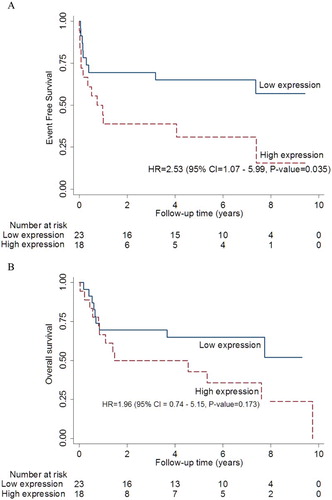
Table 3. The multivariate analyses of the risk factors for poor survival in DLBCL.
ERβ expression and outcomes in primary extra-nodal lymphoma group
In primary extra-nodal DLBCL, the mean ERβ expression in the good outcome group was 64.1 ± 41.6% (Median 50%, IQR 95%), while the mean expression in the poor outcome group was 29.9 ± 40.2% (Median 5%, IQR 80%). The difference between the two groups was significant (P = 0.006) by Mann–Whitney U-test.
The comparison of the baseline characteristics between low (<25%) and high (≥25%) expression groups is shown in . There was no statistical difference in age, sex, performance status, advanced stages of diseases, elevated LDH, the proportion of high–intermediate to high IPI risk or aaIPI, CR rate after R-CHOP and the proportion of non-GCB subtype of DLBCL, as determined by immunohistochemistry using Hans criteria.
Table 4 The characteristics of extra-nodal DLBCL patients (N = 31) divided by the expression of ERβ into low expression (<25%) and high expression (≥25%).
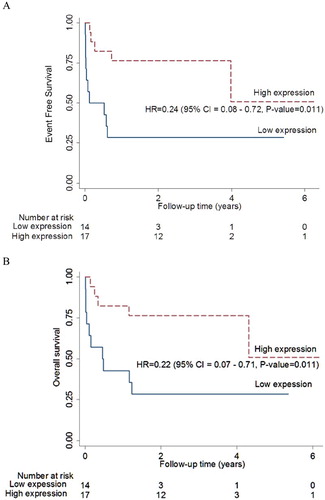
The high ERβ expression was associated with the longer EFS with the HR of 0.24 (95%CI 0.08–0.72, P = 0.011) at the median follow-up time of 21 months, as shown in (A). In addition, higher ERβ expression was also significantly associated with better OS as in (B). High IPI showed a trend toward poorer outcomes with the HR of 3.44 (95%CI 0.77–15.42, P = 0.107).
Figure 3. EFS of the extra-nodal DLBCL. (A) High ERβ expression was associated with longer EFS in extra-nodal DLBCL. (B) High ERβ expression was associated with longer OS in extra-nodal DLBCL.
Using multivariate analysis, only low ERβ expression was associated with shorter EFS and shorter OS in extra-nodal DLBCL ().
Discussion
The current study found that the majority of the DLBCL tissues, both nodal and extra-nodal, expressed variable degrees of ERβ1 protein (ranging from 0 to 100%) consistent with previous data showing that normal B lymphocytes expressed the mRNA of ERβ and no or very low ERα [Citation9,Citation18]. Consistent with previous data in Caucasians, the majority of DLBCL in Thai patients displayed ERβ [Citation17]. The ERβ antibody used in the study was specific to ERβ1 isoform and it was the same clone as the previous study [Citation17]. Unlike the other alternatively splicing isoforms, this isoform is the full-length protein that is capable of ligand binding [Citation19], suggesting that the ER in DLBCL may be responsive to estrogens or ERβ agonists.
We proposed in this study that the degree of expression could be represented as percentage of positive cells in order to be determined reproducibly. The 25% expression could be used as cut-off points for predicting outcomes in nodal and extra-nodal DLBCL. These cut points remain to be validated in a larger study. Expression of ERβ can be counted by either the manual counting method or the automated counting method. At least 500 cells were counted in total. In our pilot study, both methods showed a moderate degree of correlation with a coefficient of 0.426 (P = 0.01). However, the automated count method could not differentiate between the lymphoma cells and the remaining normal cells. Previous reports suggested that ERβ expression in tumor cells was responsible for the effects [Citation15]. Therefore, the manual count by the experienced hematopathologist was likely to be more accurate and was chosen to enumerate the expression of ERβ.
The mechanisms of variable ERβ expression among different cases and among different lymphoma cells in the same case remain to be determined. Notably, peripheral blood mononuclear cells from normal volunteers expressed variable degrees of ERs [Citation13]. Therefore, these variations may be the inherent property of lymphoid cells unrelated to oncogenic events. The variable expression of ERα in lymphoid cells has been found to be under the epigenetic control by promoter methylation [Citation8]. Whether this is also true for ERβ remains to be investigated. In this study, the degrees of expression were not significantly correlated with any baseline characteristics including age, sex, hormonal status, primary sites (Nodal vs. extra-nodal lymphoma) or cellular origin (GCB vs. Non-GCB) of DLBCL.
In nodal DLBCL treated with R-CHOP chemotherapy, high ERβ protein expression (≥25%) was related to poor EFS. This is consistent with the recent report by Hasni et al. [Citation17]. On the other hand, low ERβ protein expression was significantly related to poor EFS in primary extra-nodal DLBCL. This effect is independent of the IPI group in a multivariate analysis. Therefore, ERβ is a potentially novel biomarker for prognosis in DLBCL. Confirmatory studies in larger numbers of patients are warranted. The better prognosis of high ERβ expression in extra-nodal lymphoma is consistent with previous animal studies. Notably, the mouse xenograft models are a form of extra-nodal lymphoma [Citation17].
There are two types of prognostic factors in DLBCL. First, IPI is clinically determined to reflect both the host factors (age and performance status) and tumor burden (stage, LDH, extra-nodal involvements and performance status) [Citation20]. The second type is the tumor biology including cellular origin from gene expression profiling [Citation21] and multiplicity of genetic lesions (double-hit or triple-hit lymphoma) from FISH studies [Citation22]. ERβ is likely one of the lymphoma biology markers and may be used as a prognostic factor complementary to IPI as it can be evaluated easily using routine immunohistochemistry. In this study, 18 samples were evaluated for MYC and BCL2 expression by immunohistochemistry and 7 (3 9 %) of them were double-protein-expression lymphoma that had a worse prognosis [Citation23]. Within this small sample size, the MYC/BCL2 double expression showed no association with the prognostic groups, as determined by ERβ expression (data not shown).
The reason for the opposite effects on ERβ expression in nodal vs. extra-nodal DLBCL is still unclear. Previous studies reported that nodal vs. extra-nodal DLBCL had distinct genotypes [Citation24] and gene expression profiles [Citation25], suggesting different signaling pathways. ERβ expression, therefore, may affect different molecules in different primary sites of lymphoma. Notably, the gene expression in the microenvironment of DLBCL is important for lymphoma growth [Citation26]. Therefore, there may be dissimilar biological effects of ERβ in different tumor microenvironments especially when concomitant immunotherapy was given.
It is unclear why the high ERβ expression in nodal DLBCL is associated with the poor prognosis despite the potential tumor-suppresser role of ERβ. Animal studies showed that artificial expression of ER β receptor to cancer cells resulting in tumor suppression and exogenous estrogen potentiated this effect [Citation27–29]. Nevertheless, over 90% of subjects in this study were either men or post-menopausal women. Therefore, ERβ in DLBCL from this study is likely to be inactive due to the absence of estrogen. Among six premenopausal women in this study, four of them had nodal lymphoma. Two (50%) premenopausal nodal DLBCL with high ERβ expression had poor outcomes and the other two (50%) with low expression showed good outcomes. Two premenopausal women with extra-nodal DLBCL (one each had high and low ERβ expression) showed good outcomes. Owing to a small number of patients, the effect of endogenous estrogen on the prognosis is still inconclusive.
The effects of ERβ on DLBCL biology deserved future investigations. Because the selective ERβ agonist is available, this receptor has potentials to be a new targeted therapy. ERβ1 expression may be a response predictor if the ERβ receptor agonist is use in DLBCL patients in the future. This study is limited by the retrospective nature and a small sample size. Confirmation by a larger prospective study is needed.
In summary, the majority of the DLBCL cells expressed variable degrees of ERβ protein. The higher percentages of expression were associated with a poor prognosis in nodal DLBCL, but related with good prognosis in extra-nodal DLBCL.
Acknowledgments
The authors are very grateful to the nurses and staff of the hematology division and pathology department for their help.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ponlapat Rojnuckarin http://orcid.org/0000-0001-7912-1996
Additional information
Funding
References
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508
- Nelson RA, Levine AM, Bernstein L., et al. Reproductive factors and risk of intermediate- or high-grade B-cell non-Hodgkin’s lymphoma in women. J Clin Oncol. 2001;19:1381–1387 doi: 10.1200/JCO.2001.19.5.1381
- Lee JS, Bracci PM, Holly EA. Non-Hodgkin lymphoma in women: reproductive factors and exogenous hormone use. Am J Epidemiol. 2008;168:278–288. doi: 10.1093/aje/kwn119
- Lu Y, Wang SS, Sullivan-Halley J, et al. Oral contraceptives, menopausal hormone therapy use and risk of B-cell non-Hodgkin lymphoma in the California teachers study. Int J Cancer. 2011;129:974–982 doi: 10.1002/ijc.25730
- Kane EV, Roman E, Becker N, et al. Menstrual and reproductive factors, and hormonal contraception use: associations with non-Hodgkin lymphoma in a pooled analysis of InterLymph case-control studies. Ann Oncol. 2012;23:2362–2374 doi: 10.1093/annonc/mds171
- Cerhan JR, Vachon CM, Habermann TM, et al. Hormone replacement therapy and risk of non-Hodgkin lymphoma and chronic lymphocytic leukemia. Cancer Epidemiol Biomarkers Prev. 2002;11:1466–1471
- Costas L, de Sanjosé S, Infante-Rivard C., et al. Reproductive factors and non-Hodgkin lymphoma: a systematic review. Crit Rev Oncol Hematol. 2014;92:181–193 doi: 10.1016/j.critrevonc.2014.07.004
- Ladikou EE, Kassi E. The emerging role of estrogen in B cell malignancies. Leuk Lymphoma. 2017;58:528–539. doi: 10.1080/10428194.2016.1213828
- Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol. 2013;375:121–129. doi: 10.1016/j.mce.2013.05.016
- Meyers MJ, Sun J, Carlson KE, et al. Estrogen receptor-β potency-selective ligands: structure−activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a
- Sun J, Baudry J, Katzenellenbogen JA, et al. Molecular basis for the subtype discrimination of the estrogen receptor-β-selective ligand, diarylpropionitrile. Mol Endocrinol. 2003;17:247–258. doi: 10.1210/me.2002-0341
- Yakimchuk K, Iravani M, Hasni MS, et al. Effect of ligand-activated estrogen receptor β on lymphoma growth in vitro and in vivo. Leukemia. 2011;25:1103–1110. doi: 10.1038/leu.2011.68
- Yakimchuk K, Norin S, Kimby E, et al. Up-regulated estrogen receptor β2 in chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:139–144. doi: 10.3109/10428194.2011.605187
- Talaber G, Yakimchuk K, Guan J, et al. Inhibition of estrogen biosynthesis enhances lymphoma growth in mice. Oncotarget 2016;7:20718–20727. doi: 10.18632/oncotarget.7843
- Yakimchuk K, Hasni MS, Guan J, et al. Inhibition of lymphoma vascularization and dissemination by estrogen receptor β agonists. Blood. 2014;123:2054–2061. doi: 10.1182/blood-2013-07-517292
- Roemer K, Pfreundschuh M. How do estrogens control lymphoma? Blood. 2014;123:1980–1981. doi: 10.1182/blood-2014-02-554691
- Hasni MS, Berglund M, Yakimchuk K, et al. Estrogen receptor β1 in diffuse large B-cell lymphoma growth and as a prognostic biomarker. Leuk Lymphoma. 2017;58:418–427. doi: 10.1080/10428194.2016.1193853
- Phiel KL, Henderson RA, Adelman SJ, et al. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007
- Taylor SE, Martin-Hirsch PL, Martin FL. Oestrogen receptor splice variants in the pathogenesis of disease. Cancer Lett. 2010;288:133–148. doi: 10.1016/j.canlet.2009.06.017
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545
- Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879
- Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol 2015;16:e555–e567. doi: 10.1016/S1470-2045(15)00005-4
- Al-Humood SA, Al-Qallaf AS, Alshemmari SH, et al. Genotypic and phenotypic differences between nodal and extranodal diffuse large B-cell lymphomas. J Histochem Cytochem. 2011;59:918–931. doi: 10.1369/0022155411417309
- Jehan Z, Siraj AK, Abubaker J, et al. Distinct gene expression profiles: nodal versus extranodal diffuse large B-cell lymphoma. Oncology. 2008;75:71–80. doi: 10.1159/000155144
- Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885
- Hartman J, Edvardsson K, Lindberg K, et al. Tumor repressive functions of estrogen receptor in SW480 colon cancer cells. Cancer Res 2009;69:6100–6106. doi: 10.1158/0008-5472.CAN-09-0506
- Li H, Tu Z, An L, et al. Inhibitory effects of ERβ on proliferation, invasion, and tumor formation of MCF-7 breast cancer cells – prognostication for the use of ERβ-selective therapy. Pharm Biol. 2012;50:839–849. doi: 10.3109/13880209.2011.637506
- Tu Z, Ma Y, Tian J, et al. Estrogen receptor β potentiates the antiproliferative effect of raloxifene and affects the cell migration and invasion in HCT-116 colon cancer cells. J Cancer Res Clin Oncol. 2012;138:1091–1103. doi: 10.1007/s00432-011-1145-3