ABSTRACT
Objectives: Severe aplastic anemia (SAA) patients receive more red blood cell (RBC) transfusions as supportive management. We aim to clarify the associations between transfusion history or pre-transplantation serum ferritin (SF) and the overall survival of allogeneic hematopoietic stem cell transplantation (allo-HSCT) among SAA patients.
Material and methods: We retrospectively investigated 96 SAA patients undergoing allo-HSCT, and grouped them according to pre-transplantation duration. Pre-transplantation SF, transfused units and other iron-related parameters were collected. Comparisons in transplantation outcomes and complications were made in groups with different SF levels and different transfusion histories.
Results: Among the 96 SAA patients, 45 patients received transplantation within 2 months after diagnosis (short-term pre-transplantation period), and the rest of the patients had long-term pre-transplantation treatment. Among the patients with short-term pre-transplantation treatment, a higher risk of death was seen in the high-ferritin group (p < 0.05). Elevated SF also predicted a trend in incidence of higher bloodstream infection (p = 0.108). Significant correlations were observed between pre-transplantation SF and infection incidence, as well as transfusion history. However, for patients with longer pre-transplantation duration, transfusion history was associated with worse outcome (p = 0.026), in terms of higher incidence of acute graft versus host disease (p = 0.048). High SF was only significantly associated with prolonged RBC transfusion dependence post-transplantation (p = 0.044).
Discussion and conclusions: Transfusion history was a stronger predictor of outcome than SF in patients undergoing transplantation more than 2 months after diagnosis.
Introduction
There have been numerous studies that have revealed the association between iron overload and allogeneic hematopoietic stem cell transplantation (allo-HSCT), and most investigators preferred serum ferritin (SF) as an indicator of iron burden, as well as an acute phase reactant [Citation1]. However, controversies still exist over whether high-ferritin equals iron overload, and whether it leads to poor outcome [Citation3].
Patients with severe aplastic anemia (SAA) for the past 20 years have achieved better treatment outcomes, since immunosuppressive therapy (IST) and allo-HSCT began to be widely applied [Citation4]. However, these patients require massive red blood cell (RBC) transfusion and are at risk for iron overload. As far as we know, there is no sizable clinical summary of the impact of SF on the outcome in Chinese patients with aplastic anemia (AA) undergoing allo-HSCT. Despite that, the associations between SF and transfusion history as well as inflammatory markers in SAA patients are not clear. To obtain a better understanding of the unsolved problems, we carried out a retrospective study to analyze the role of SF in different populations of AA patients.
Materials and methods
Patients
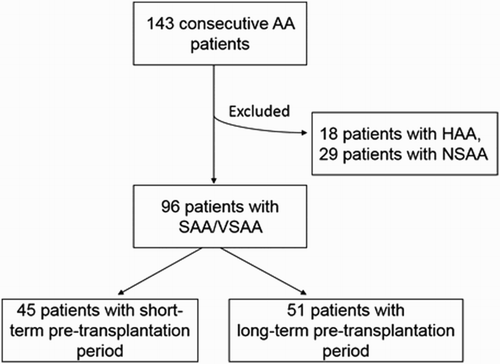
We retrospectively analyzed 143 consecutive patients with AA who underwent allo-HSCT between October 2008 and January 2016 in the Department of hematopoietic stem cell transplantation at the Blood Disease Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences (PUMC&CAMS). Nighty-six patients were included in the present study, and a total of 47 patients were excluded for 18 patients with hepatitis-associated aplastic anemia and 29 patients with non-severe aplastic anemia. The myeloablative conditioning regimens we used were all non-total body irradiation (TBI)-based regimens, basically consisting of cyclophosphamide (Cy), fludarabine (Flu), and antithymocyte globulin (ATG). Busulphan (Bu) and Ara-C were offered to patients with long-term pre-HSCT therapy. In addition, we used two kinds of ATG, including rabbit anti-human thymocyte globulin and pig antithymocyte globulin (ALG). The dose of rabbit ATG was 8–12.5 mg/kg and of ALG was 64–100 mg/kg. Calcineurin inhibitors (cyclosporine [CsA] or tacrolimus) with or without short-term methotrexate treatment were used for graft versus host disease (GVHD) prophylaxis in most patients. Fluconazole prophylaxis against fungal infection, acyclovir prophylaxis against herpes simplex virus infection, and sulfamethoxazole/trimethoprim prophylaxis against Pneumocystis jiroveci infection were used. Human leukocyte antigen (HLA)-matching for donor selection was based on HLA high resolution typing for HLA-A, B, C, DRB1, DQB1 antigen. This retrospective study was conducted in accordance with the Helsinki declaration and approved through the ethical review process by the institutional review board. All patients provided written informed consent.
Study design
All clinical characteristics including gender, age, disease severity, RBC transfusion history, pre-HCT infection history, conditioning regimen, stem cell source, and mononuclear cells and CD34 + cell number were all collected from the case chart. Pre-transplantation SF and other related parameters such as serum iron (SI), total iron binding capacity, unsaturated iron binding capacity, iron saturation ratio, soluble transferrin receptor (sTfR), and interleukin 6 (IL-6) were routinely measured before the conditioning regimen. Post-transplantation follow-up information, such as survival, acute GVHD (aGVHD) or chronic GVHD (cGVHD) incidence, and infections were obtained from the case chart and physician’s database routinely recorded. Patients were divided into two cohorts on the basis of time from diagnosis to transplantation. And comparison was made between high and low pre-HCT ferritin or transfusion groups within each cohort.
Definitions
High-ferritin was defined as SF levels greater than or equal to 1000 ng/ml. Rejection includes both primary rejection and secondary rejection, the former is defined as failure to engraft by day 28 post-transplantation, and secondary rejection refers to loss of graft function. Neutrophil recovery was defined as an absolute neutrophil count of greater than or equal to 0.5 × 109/L, and platelet engraftment was defined as a platelet count of greater than or equal to 20 × 109/L without transfusion support. The severity of acute GVHD and cGVHD was graded according to the clinical consensus criteria. Infection was graded using the rank of Common Terminology Criteria for Adverse Events. Infection diagnosed by pathogen needed both evidence of pathogen isolated and diagnosis by physician. Overall survival (OS) was calculated from the day of transplantation, with patients alive at the time of the last follow-up being administratively censored.
Statistical analysis
The duration of follow-up was the time from transplant to the last assessment for survivors. Variables related to the patients and post-transplantation complications were compared between the groups with Fisher’s exact test for categorical variables and t-test for continuous variables. Correlations between various parameters were estimated using the Spearman correlation coefficient. Probabilities of OS were calculated with the Kaplan–Meier method.
Results
Patient characteristics
This retrospective observational study included 96 patients with SAA who underwent allo-HSCT. The median age was 19.5 years (range: 3–49), and the median duration from diagnosis to transplantation was 2.5 months (range: 0.7–48). Almost half of the patients (45, 46.9%) received transplantation in early-stage disease within 2 months after diagnosis. The comparison of demographic and clinical characteristics between patients with different duration from diagnosis to transplantation is summarized in . The age, disease severity, treatment before transplantation, and stem cell transplantation type were significantly different between the two cohorts. It is noteworthy that the patients who were younger, who did not receive ATG therapy, or who had HLA-matched sibling donors had more opportunities to conduct transplantation in early-stage disease. shows the iron-related parameters of the two cohorts. The mean SF was 643.33 ng/ml (±434.818) and 1036.68 ng/ml (±792.522), respectively, and the difference was significant (p < 0.05). Consistently, the RBC-transfused units and SI were also significantly higher in patients with long duration from diagnosis to transplantation. It can be easily understood that patients with long duration prior to HSCT required more RBC-transfused units, which induced an increase in SF and SI levels. Additionally, it is also interesting to notice the difference of IL-6 levels between the two cohorts (94.07 ± 67.681 vs. 58.74 ± 70.043, p = 0.027).
Table 1. Basic characteristics between patients with different durations from diagnosis to transplantation.
Table 2. Comparison of SF and its related parameters between patients with different duration of pre-HSCT.
Impact of pre-HSCT SF and transfusion history on transplantation outcome
The 96 patients were followed up for a median of 24.5 months (range 0.7–99.5). A total of seven patients died due to severe complications of HSCT, and the OS rates in patients with short- or long-term pre-HSCT therapy were 93.33% and 92.16% (p = NS), respectively.
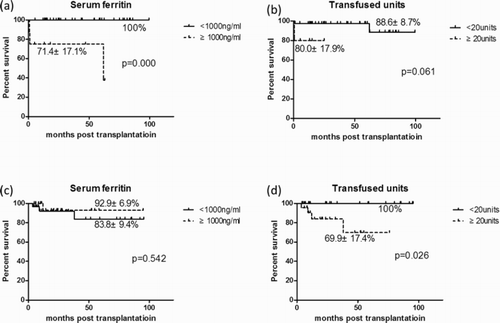
The OS of both groups of patients with high- and low-ferritin in early-stage disease is shown in (a). A higher risk of death was seen in the high-ferritin group (p < 0.05). Two patients died of severe infection and hemorrhage within one month post-transplantation, and the other patient died of cGVHD at 3 years post-transplantation. However, the difference between patients with different transfused RBC units was not significant ((b)).
(c) shows that for patients with long-term pre-HSCT therapy, the high-ferritin group did not show poor outcome, even with a trend of slightly better outcome (83.8 ± 9.4% vs. 92.9 ± 6.9%, p = 0.524). Only one patient with a ferritin level ≥1000 ng/ml died of severe infection. Contrarily, patients with excessive transfusion history displayed a significantly worse outcome (100% vs. 69.9 ± 17.4%, p = 0.026) ().
Figure 2. OS in patients with short-term and long-term pre-HSCT therapy. (a) Survival analysis of patients with high and low SF levels; (b) survival analysis of patients with transfusion of ≥20 or <20 U; (c) survival analysis of patients with high and low SF levels; (d) survival analysis of patients with transfusion of ≥20 or <20 U.
Impact of pre-HSCT SF and transfusion history on stem cell engraftment
We used the time to neutrophil engraftment, and platelet and RBC transfusion independence for the evaluation of hematologic recovery. No patients experienced primary rejection in our study; 5/45 patients with short-term pre-HSCT periods and 7/51 patients with long-term pre-HSCT periods were diagnosed with secondary rejection. Otherwise, no significant difference was observed between different SFs and transfusion histories ().
Table 3. Treatment outcome of patients with short-term pre-HSCT periods (≤2 months).
For patients whose duration from diagnosis to transplantation was shorter than 2 months, the high-ferritin group and excessive transfusion group showed similar trends in prolonged duration of RBC transfusion independence (42.1 vs. 57.1%, p = 0.682; 41.0 vs. 66.7%, p = 0.383) (). Otherwise, among patients with longer-term pre-HSCT treatment, there was a remarkable increase in the RBC transfusion independence delay in patients with high-ferritin levels and excessive transfusion (25.8 vs. 55%, p = 0.044; 25.0% vs. 52.2%, p = 0.080).
Table 4. Treatment outcome of patients with long-term pre-HSCT periods (>2 months).
Impact of pre-HSCT SF and transfusion history on GVHD
In patients with shorter duration from diagnosis to transplantation, the incidence of aGVHD in the high-ferritin group (42.9%) was slightly higher than in the low-ferritin group (36.8%). But patients with SF levels < 1000 ng/ml showed higher incidence of III-IV aGVHD (5.9% vs. 0%). Among the patients with longer pre-transplantation duration, the incidence of III-IV aGVHD in the low- and high-ferritin groups was 16.2 and 5%, respectively. However, patients with transfusion of ≥20 U had significant higher incidence of aGVHD (39.3% vs. 69.6%, p = 0.048) ().
For patients receiving transplantation at the early stage, incidence of cGVHD in both low- and high-ferritin groups was similar (31.6% vs. 28.6%), but more patients in the high-ferritin group and more-transfusion group had extensive cGVHD incidence (15.8% vs. 28.6%, p = 0.590; 15.4% vs. 33.3%, p = 0.286). Otherwise, in patients with long pre-transplantation duration, the incidence of cGVHD in high-ferritin patients was higher (45% vs. 29.0%, p = 0.238), and extensive cGVHD incidence was similar (19.4% vs. 15.0%). However, there were more patients with cGVHD as well as extensive cGVHD in the excessive transfusion group (25.0% vs. 47.8%, p = 0.141; 7.1% vs. 30.4%, p = 0.061) ().
Impact of pre-HSCT SF on infection
Among the patients with short-term pre-HSCT periods, patients in the low- and high-ferritin groups had similar incidences of bacterial infection, fungal and viral infections (52.6% vs. 57.1%, p = 1.000; 23.7% vs. 14.3%, p = 1.000; 42.9% vs. 34.2%, p = 0.686). In addition, the incidence of cytomegalovirus (CMV) infection was also similar in the two groups (23.68% vs. 28.75%). However, patients with high SF levels and more transfusion had higher incidence of severe infection (5.3% vs. 28.6%, p = 0.108; 7.7% vs. 16.7%, p = 0.448). 28.6% (2/7) patients in the high-ferritin group and 5.3% (2/38) patients in the low-ferritin group experienced bacterial bloodstream infection (BSI) (p = 0.108). For fungus infection, 14.3% (1/7) in the high-ferritin group and no patients in the low-ferritin group developed fungemia, but it is not significantly different ().
It was a different situation in patients who did not receive HSCT within 2 months after diagnosis that patients with high SF did not show higher incidence of bacterial and fungal infections (71% vs. 65%, p = 0.760; 22.6% vs. 30%, p = 0.743), except for viral infection (38.7% vs. 55%, p = 0.388). Patients with SF levels ≥1000 ng/ml have higher incidence of CMV infection (50.0% vs. 35.48%, p = NS). However, patients receiving more RBC transfusion pre-HSCT experienced almost three times risk of BSI (7.1% vs. 21.7%, p = 0.221) ().
SF and its related parameters
We investigated the associations between pre-HSCT SF and other potential related parameters, including RBC transfusion units, iron-related parameters, and inflammation history. Among the patients who underwent allo-HSCT in early-stage disease, RBC transfusion units (7.46 ± 7.11 vs. 14.33 ± 10.55, p = 0.047) and pre-HSCT infection incidence (47.4% vs. 100%, p = 0.012) were both significantly different between patients with different SF levels. In contrast, for patients with long-term pre-HSCT treatment, the sTfR level showed a significant difference (0.60 ± 0.332 vs. 0.31 ± 0.336, p = 0.011) (Table SIV and ).
Table 5. Correlation analysis between SF and its related parameters in patients with different durations of pre-HSCT.
We further performed the correlation analysis using the continuous variable of SF, instead of the categorical variable (). In short-term pre-HSCT treatment cohort, SF was associated with both pre-HSCT infection incidence (r = 0.320, p = 0.034) and RBC transfusion (r = 0.388, p = 0.011). Otherwise, for patients having longer duration from diagnosis to transplantation, SF has a stronger correlation with transfusion (0.510, p = 0.001). Furthermore, SF itself was strongly negatively correlated to sTfR (−0.543, p = 0.000).
Discussion
Our primary objective is to determine whether pre-HSCT SF and transfusion history could predict the complications and outcomes of allo-HSCT in SAA patients. In our retrospective analysis, we grouped these patients according to the duration from diagnosis to transplantation. In our institution, if the SAA patient has an HLA-identical sibling donor, HSCT can proceed as soon as possible, mostly within the 2 months after diagnosis. Otherwise, we recommend IST therapy and try to identify alternative donors, including unrelated donors. Most of those patients in the absence of matched HLA sibling donors receive transplantation more than 2 months after diagnosis, as assessment of response to IST is usually made at 3–6 months. Hence, we used the 2-month time point to distinguish between long and short pre-HSCT duration. We assumed that those two populations of patients received different pre-transplantation therapy, including different amounts of RBC transfusion, so the iron burden and SF would also be different. The secondary objective is to investigate the relationship between SF and RBC transfusion among patients with different durations of pre-HSCT therapy, and determine whether SF is a better predictor than transfusion history.
The role of pre-HSCT SF in the outcome remains controversial, although it has been widely studied in patients with both benign and malignant hematologic diseases over the last 20 years [Citation5]. During the early 10 years, numerous studies have shown that elevated SF prior to HSCT is detrimental to OS with an increase in non-relapse mortality, as a consequence of iron overload toxicity [Citation6–10]. However, the enrollments in many previous articles always included various diseases, including AA, myelodysplastic syndromes, and acute or chronic hematologic malignancies [Citation3,Citation11,Citation12]. As it is still unknown whether SF plays the same role in different hematologic diseases, and patients with those different kinds of diseases also received different amounts of RBC transfusion, we enrolled only SAA patients this time. Additionally, we grouped patients according to different interventions from diagnosis to transplantation. We think this kind of group can provide a better understanding of the role of SF and transfusion predicting HSCT outcome for AA patients, avoiding interpretations of patients’ heterogeneity. As we have showed, there were significant differences mainly in transfusion history, SF, and IL-6 between the two groups. It can be well understood that very SAA and SAA patients undergoing HSCT more than 2 months after diagnosis received more RBC transfusion as supportive management.
Even the conclusion has not been well established [Citation13–15]; there are increasing reports that SF may be a better surrogate of disease status which would be associated with HSCT outcome with its poor specificity, rather than LIC or other iron-related parameters [Citation1,Citation16,Citation17]. According to our results, SF is a different surrogate from transfusion history in predicting HSCT outcome for SAA patients. It is interesting to find that in patients with short-term pre-HSCT periods, high ferritin had a negative impact on OS in terms of a trend of higher incidence of BSI and slight increase of incidence of extensive cGVHD. Our results indicating the association between SF and high BSI incidence are in agreement with previous studies showing hyperferritinemia raises the risk of severe infection [Citation1,Citation7]. We also reported that high SF was not related to higher incidence of aGVHD, which is supported by previous articles. M. G. Seidel and his colleagues found that high SF pre-HSCT was not a good biomarker of aGVHD, especially III-IV aGVHD [Citation1][Citation2].
However, in our study, excessive pre-HSCT transfusion history, not SF, was detrimental to OS, because it significantly increased the incidence of aGVHD in those patients. In a recent published paper, Lee et al. used RBC units transfused to evaluate HSCT outcome in AA patients. Their results indicated that a history of higher pre-transplantation transfusion was associated with decreased OS and higher incidence of aGVHD [Citation18]. Compared with SF, transfused RBC was considered to be a better indicator of iron burden, because each unit of RBC transfusion added approximately 200 mg iron in the body [Citation19]. Furthermore, there are reports about the relationship between transfused units and SF [Citation20]. Considering the poor specificity of SF, transfusion history would be a better prognostic factor for allo-HSCT outcome in patients with long-term transfusion as supportive treatment [Citation21].
Due to poor specificity of SF [Citation1,Citation22], we hypothesized that part of SF plays the role of acute inflammatory reactant in the early stage of SAA. The evidence supporting our assumption was the significantly elevated levels of IL-6 and slight correlation between SF and infection incidence. It has been reported that, after analyzing the iron status in 550 patients newly diagnosed with SAA, around 10% of patients presented with SF levels ≥1000 ng/ml at new diagnosis, without a history of transfusion [Citation23]. There are also a few studies reporting the relationship between pre-HSCT inflammation and outcome without established conclusion. Bazuaye et al. documented that an increase in pre-HSCT CRP was also a good predictor of OS [Citation24]. But Sakamoto et al. retrospectively studied 220 patients and found that an elevated serum CRP level was not a strong risk factor for overall mortality [Citation25]. Interestingly, in patients with long-term pre-HSCT therapy, SF, instead of transfusion history, correlated with sTfR (r = −0.543). sTfR was reported as an indicator of bone marrow failure severity and a key factor predicting AA treatment outcome [Citation26]. It could be explained by the low level of sTfR that patients in the high-SF group had higher incidence of transfusion independence delay.
Conclusions
High SF pre-HSCT, which correlated with both infection and transfusion, predicted inferior OS in patients with short-term pre-HSCT treatment. But for patients with long-term treatment of pre-HSCT, transfusion history can be a more powerful indicator of higher incidence of aGVHD and worse OS. As a consequence, for patients who would chose IST therapy or waited for donor identification, excessive RBC transfusion should be avoided, and iron chelation therapy could be taken into consideration before transplantation.
Acknowledgement
We thank all patients and the data collection team who participated in this study. We really appreciate the help from Liu Jie who is responsible for the follow-up database. We also thank Jianfeng Yao for help at the early stage of data collection, and all the family that provided us access to their facilities to complete this study. A special thanks to Kutay Karatepe for his critical comments and proofreading.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Donglin Yang http://orcid.org/0000-0003-4056-4743
References
- Armand P, Kim HT, Virtanen JM, et al. Iron overload in allogeneic hematopoietic cell transplantation outcome: a meta-analysis. Biol Blood Marrow Transplant. 2014;20(8):1248–1251. PubMed PMID: 24769316; PubMed Central PMCID: PMC4099413. doi:10.1016/j.bbmt.2014.04.024.
- Grossekatthofer M, Guclu E. D, Lawitschka A, et al. Ferritin concentrations correlate to outcome of hematopoietic stem cell transplantation but do not serve as biomarker of graft-versus-host disease. Ann Hematol. 2013;92(8):1121–1128. doi: 10.1007/s00277-013-1737-x
- Pileggi C, Di Sanzo M, Mascaro V, et al. Role of serum ferritin level on overall survival in patients with myelodysplastic syndromes: results of a meta-analysis of observational studies. PloS One. 2017;12(6):e0179016. Epub 2017/06/18. PubMed PMID: 28622367; PubMed Central PMCID: PMCPmc5473533. doi:10.1371/journal.pone.0179016.
- Peinemann F, Bartel C, Grouven U. First-line allogeneic hematopoietic stem cell transplantation of HLA-matched sibling donors compared with first-line ciclosporin and/or antithymocyte or antilymphocyte globulin for acquired severe aplastic anemia. Cochrane Database Syst Rev. 2013;(7):Cd006407. Epub 2013/07/25. PubMed PMID: 23881658. doi:10.1002/14651858.CD006407.pub2.
- Trottier BJ, Burns LJ, DeFor TE, et al. Association of iron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122(9):1678–1684. Epub 2013/06/20. PubMed PMID: 23777771; PubMed Central PMCID: PMCPmc3953015. doi:10.1182/blood-2013-04-499772.
- Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95(3):476–484. Epub 2009/11/12. PubMed PMID: 19903678; PubMed Central PMCID: PMCPmc2833079. doi:10.3324/haematol.2009.011429.
- Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42(12):799–805. Epub 2008/09/03. PubMed PMID: 18762767. doi:10.1038/bmt.2008.262.
- Platzbecker U, Bornhauser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS). Biol Blood Marrow Transplant. 2008;14(11):1217–1225. Epub 2008/10/23. PubMed PMID: 18940675; PubMed Central PMCID: PMCPmc2759308. doi:10.1016/j.bbmt.2008.08.006.
- Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34(6):723–727. Epub 2009/12/01. PubMed PMID: 19944463. doi:10.1016/j.leukres.2009.10.028.
- Atilla E, Toprak SK, Demirer T. Current review of iron overload and related complications in hematopoietic stem cell transplantation. Turk J Haematol. 2017;34(1):1–9. Epub 2016/12/14. PubMed PMID: 27956374; PubMed Central PMCID: PMCPmc5451667. doi:10.4274/tjh.2016.0450.
- Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–4588. Epub 2007/01/20. PubMed PMID: 17234738; PubMed Central PMCID: PMCPmc1885508. doi:10.1182/blood-2006-10-054924.
- Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146(3):310–316. Epub 2009/06/11. PubMed PMID: 19508292. doi:10.1111/j.1365-2141.2009.07774.x.
- Wermke M, Schmidt A, Middeke JM, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Cancer Res. 2012;18(23):6460–6468. Epub 2012/09/20. PubMed PMID: 22991415. doi:10.1158/1078-0432.ccr-12-1683.
- Armand P, Kim HT, Rhodes J, et al. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):852–860. Epub 2010/09/22. PubMed PMID: 20854920; PubMed Central PMCID: PMCPmc3954514. doi:10.1016/j.bbmt.2010.09.006.
- Virtanen JM, Itala-Remes MA, Remes KJ, et al. Prognostic impact of pretransplant iron overload measured with magnetic resonance imaging on severe infections in allogeneic stem cell transplantation. Eur J Haematol. 2013;91(1):85–93. Epub 2013/04/17. PubMed PMID: 23586843. doi:10.1111/ejh.12123.
- Tanaka M, Kanamori H, Matsumoto K, et al. Clinical significance of pretransplant serum ferritin on the outcome of allogeneic hematopoietic SCT: a prospective cohort study by the Kanto study group for cell therapy. Bone Marrow Transplant. 2015;50(5):727–733. Epub 2015/03/03. PubMed PMID: 25730191. doi:10.1038/bmt.2015.17.
- Vaughn JE, Storer BE, Armand P, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biol Blood Marrow Transplant. 2015;21(8):1418–1424. Epub 2015/04/12. PubMed PMID: 25862589; PubMed Central PMCID: PMCPmc4506728. doi:10.1016/j.bbmt.2015.04.002.
- Lee SE, Yahng SA, Cho BS, et al. Impact of pretransplant red cell transfusion on outcome after allogeneic stem cell transplantation in adult patients with severe aplastic anemia. Bone Marrow Transplant. 2016;51(10):1323–1329. Epub 2016/05/24. PubMed PMID: 27214082. doi:10.1038/bmt.2016.140.
- Porter JB. Practical management of iron overload. Br J Haematol. 2001;115(2):239–252. Epub 2001/11/13. PubMed PMID: 11703317. doi: 10.1046/j.1365-2141.2001.03195.x
- Lee JW. Iron chelation therapy in the myelodysplastic syndromes and aplastic anemia: a review of experience in South Korea. Int J Hematol. 2008;88(1):16–23. Epub 2008/07/08. PubMed PMID: 18604581; PubMed Central PMCID: PMCPmc2516545. doi:10.1007/s12185-008-0117-0.
- Estcourt LJ, Malouf R, Trivella M, et al. Restrictive versus liberal red blood cell transfusion strategies for people with haematological malignancies treated with intensive chemotherapy or radiotherapy, or both, with or without haematopoietic stem cell support. Cochrane Database Syst Rev. 2017;1:Cd011305. Epub 2017/01/28. PubMed PMID: 28128441; PubMed Central PMCID: PMCPmc5298168. doi:10.1002/14651858.CD011305.pub2.
- Hilken A, Langebrake C, Wolschke C, et al. Impact of non-transferrin-bound iron (NTBI) in comparison to serum ferritin on outcome after allogeneic stem cell transplantation (ASCT). Ann Hematol. 2017;96(8):1379–1388. Epub 2017/06/07. PubMed PMID: 28585071. doi:10.1007/s00277-017-3034-6.
- Jin P, Wang J, Li X, et al. Evolution of iron burden in acquired aplastic anemia: a cohort study of more than 3-year follow-up. Int J Hematol. 2015;101(1):13–22. Epub 2014/11/29. PubMed PMID: 25430083. doi:10.1007/s12185-014-1708-6.
- Bazuaye GN, Buser A, Gerull S, et al. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2012;47(1):60–64. doi: 10.1038/bmt.2011.13
- Sakamoto S, Kawabata H, Kanda J, et al. Differing impacts of pretransplant serum ferritin and C-reactive protein levels on the incidence of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2013;97(1):109–116. doi: 10.1007/s12185-012-1229-0
- Speeckaert MM, Speeckaert R, Delanghe JR. Biological and clinical aspects of soluble transferrin receptor. Crit Rev Clin Lab Sci. 2010;47(5–6):213–228. Epub 2011/03/12. PubMed PMID: 21391831. doi:10.3109/10408363.2010.550461.