ABSTRACT
Objective: High altitude is characterized by low oxygen pressure, resulting in multiple adaptive responses. Tibetans who have lived in the plateau for thousands of years have developed unique phenotypes, such as downregulation of the HIF pathway through EPAS1 and EGLN1 gene mutation. However, the changes of hemoglobin–oxygen affinity under hypoxia environment remain elusive.
Methods: A blood cell analyzer and a blood oxygen analyzer were used to conduct routine blood tests and measure the oxygen affinity P50 in in the Han population that rapidly entered the plateau (for 3–7 days), the plateau-acclimatized Han population (residing for 30 days on the plateau), the plateau Han population (more than 10 years on the plateau), and the Tibetan population.
Results: The Han population that rapidly entered the plateau had increasing higher P50 values, RBCs counts and hemoglobin (HGB) levels, while the acclimatized Han population, the plateau Han population and Tibetan all had significantly lower P50 values. However, there were no significant differences in the RBCs counts and HGB levels between the plateau Han, Tibetan populations and the Han population of the plains.
Discussion: The adaptability of the Tibetan and plateau Han populations to the plateau was mainly due to the strong affinity of HGB for oxygen, which provided sufficient oxygen for tissues and organs.
Conclusions: The change of P50 could be a feature of the adaptation to the plateau and to avoid altitude sickness, such as high-altitude polycythemia and dyspnea.
KEYWORDS:
Introduction
The Qinghai-Tibet Plateau (>3500 m) is known for its abundant sunshine, strong ultraviolet light, dry climate and thin air, and its oxygen content is only 60% of that of the plains [Citation1]. The low oxygen pressure has a huge impact on the physiological function of populations who have lived on the plains for generations and recently entered the plateau, but Tibetans who have inhabited locations at an altitude of approximately 4000 m for generations present strong adaptability to the plateau [Citation2]. When people from the plains rapidly enter the plateau, a series of physiological changes will occur in the body, including increased red blood cell (RBCs) counts, hematocrit (Hct), hemoglobin (HGB), breathing rate and depth of breathing, lung ventilation in the respiratory system and decreased arterial oxygen saturation (SaO2) in the blood system [Citation3]. In contrast, Tibetans inhabiting the plateau do not show increased RBCs, Hct, or HGB concentrations, and the SaO2 and pulmonary arterial blood pressure of Tibetans are almost comparable to those of the who reside in the plains, suggesting that the good plateau adaptation mechanism in Tibetans involves evolutionary pressures and genetic changes [Citation4].
The oxygen affinity P50 (PO2 at which HGB is 50% saturated) is an important indicator evaluating the capability to adapt to low oxygen at high altitude. The P50 value is the partial pressure of oxygen required to achieve 50% HGB saturation measured under the standard condition (whole blood with a normal level of 2,3-diphosphoglycerate (2,3-DPG), 37°C, pH 7.40), and the average P50 is 26 ± 2 mmHg in the normal population of the plains [Citation5]. However, we have no idea on whether P50 could change during entering high-altitude environment. In the present study, we aimed to systematically compare the ability of RBCs to transport and release oxygen between the Han population of the plains, the Han population that rapidly entered the plateau (within 3–7 days of entering the plateau), the plateau-acclimatized Han population (30 days after entering the plateau), the plateau Han population (living on the plateau for more than 10 years), and the plateau Tibetan population to provide a basis for exploring the mechanism underlying the adaptive changes of RBCs in transporting and releasing oxygen as a response to high-altitude hypoxia in different populations.
Material and methods
Populations and samples
Thirty healthy male volunteers were selected as the subjects for each group and the baseline characteristics of each group were shown in . The subjects that were enrolled at the same time were divided into the Han population of the plains (inhabiting an altitude 500 m above sea level), the Han population that rapidly entered the plateau (on the 3rd and 7th days after entering the plateau at an altitude of 4300 m above sea level), the plateau-acclimatized Han population (30 days after entering the plateau an altitude of 3700 m above sea level), the plateau Han population (living on the plateau for more than 10 years at an altitude of 3700 m above sea level), and the plateau Tibetan population (inhabiting at an altitude of 3700 m above sea level; ). Peripheral blood samples with anticoagulant were collected before and after they entering plateau. This study was approved by the Institutional Review Board of General Hospital of Air Force, PLA and all volunteers signed a written informed consent.
Table 1. The baseline characteristics of each group.
Sample processing
EDTA-anticoagulated whole blood were collected and packed in EP tubes for RBCs and HGB concentration detection. After centrifugation, the precipitated RBCs were collected and then mixed with compound glycerol solution (Beijing Bode Sangte, batch number: H11022503) at a 1:1 ratio for P50 detection. Aliquots were stored at –80°C, and tests were conducted within 1 week.
RBCs counts and HGB concentration
The RBCs and HGB concentration were measured by the automatic blood cell analyzer (China Tekang Technology, model TEK3600).
Determination of oxygen affinity P50
The P50 values of each sample were measured at 37°C and pH 7.4 using the HEMOX™ blood oxygen analyzer (TCS Scientific Corp, Southampton, PA, U.S.A.).
Statistical analysis
The RBCs, HGB concentration results and P50 values were statistically analyzed using SPSS (V.20) statistical software (SPSS Inc., Chicago, IL, U.S.A.). Significant differences were calculated by one-way analysis of variance (ANOVA) followed by the Bonferroni test when performing multiple comparisons between groups and P < 0.05 indicated a statistically significant difference.
Results
RBCs counts and HGB concentration in the Tibetan and Han populations
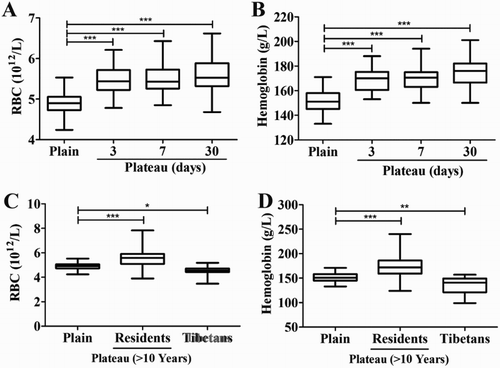
Compared to the Han population of the plains, the Han population of the plains on the 3rd day after entering the plateau (RBCs 4.8–6.2 × 1012/l, median 5.4 × 1012/l; HGB 153–188 g/l, median 170 g/l), the Han population of the plains on the 7th day after entering the plateau (RBCs 4.8–6.4 × 1012/l, median 5.4 × 1012/l; HGB 150–194 g/l, median 170 g/l), and the plateau-acclimatized Han population (RBCs 4.7–6.6 × 1012/l, median 5.5 × 1012/l; HGB 150–201 g/l, median 176 g/l) showed significantly increased RBCs and HGB levels (P < 0.05; (A,B)). There were a slightly increasing in the RBCs and HGB levels of the Han population who living in plateau for more than 10 years (RBCs 3.9–7.8 × 1012/l, median 5.6 × 1012/l; HGB 124–240 g/l, median 172 g/l), while a decreasing in the RBCs and HGB levels of the plateau Tibetan population (RBCs 3.5–5.2 × 1012/l, median 4.5 × 1012/l; HGB 99–157 g/l, median 141 g/l) were detected when compared to the Han population of the plains ((C,D)).
Figure 2. RBCs count and HGB concentration in different population. Whole blood was collected from the volunteers before and after entering the plateau, as well as Tibetan and Han population who lived in plateau for at least 10 years. RBCs count (A, C) and HGB concentration (B, D) were detected within 8 hours after blood collection. *P < 0.05, **P < 0.01 or ***P < 0.001 for subjects versus plain group.
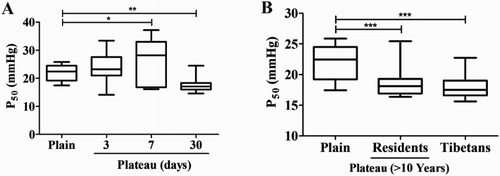
Differences in oxygen affinity P50 between the Tibetan and Han populations
The P50 value (17.44–25.86 mmHg, median 22.46 mmHg) of the Han population of the plains was slightly lower than the normal range (26.00 ± 2.00 mmHg), and that of the plain Han population on the 3rd day after entering the plateau (14.47–33.45 mmHg, median 23.22 mmHg) showed a slight increase, with no statistically significant difference compared to the Han population of the plains. The P50 value of the Han population of the plains on the 7th day after entering the plateau (16.13–37.15 mmHg, median 28.17 mmHg) was significantly higher than that of the Han population of the plains (P < 0.01), whereas that of the Han population of the plains that became acclimatized to the plateau (14.55–24.49 mmHg, median 17.08 mmHg) was significantly lower than that of the Han population of the plains (P < 0.01) (). The P50 values of the plateau Han population (P50 16.38–25.42 mmHg, median 18.10 mmHg) and the plateau Tibetan population (P50 15.62–22.72 mmHg, median 17.51 mmHg) were significantly lower than that of the Han population of the plains (P < 0.001) ().
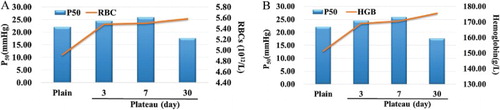
Correlation between oxygen affinity P50 and the RBCs counts and HGB concentration in the Han population
The values of P50 increased as the RBCs counts ((A)) and HGB concentration ((B)) increased on the 3rd and 7th days after the Han population of the plains entered the plateau. The RBCs counts and HGB concentration continued to increase, while the variation trend in the P50 value was opposite, with a significant decrease after the acclimation to the plateau ().
Discussion
The extreme environmental conditions of the Qinghai and Tibet Plateau (altitude > 3500 m), such as low pressure and low oxygen, can pose serious threats to the physical and mental health of people entering the plateau and may cause headaches, insomnia, dyspnea, and other plateau reactions as well as a series of physiological changes. In contrast, the Tibetan population that has lived on the plateau for generations can adapt well to the plateau environment: First, the Tibetan populations of the plateau and plains have similar HGB concentrations and SaO2, which are significantly lower than those of people from the plains who migrate to the plateau [Citation6]. Second, Tibetan people have lower hypoxic pulmonary vasoconstriction than the population of the plains, and hypoxic pulmonary vasoconstriction can cause hypoxic pulmonary hypertension and lead to pulmonary edema [Citation4]. Third, Tibetan people also have a faster blood flow velocity to ensure a normal oxygen supply to all tissues and organs [Citation7]. In this study, we analyzed the oxygen affinity P50, RBCs counts, and HGB concentration of the Han population of the plains, the Han population that rapidly entered the plateau (entered the plateau for 3 and 7 days), and the plateau-acclimatized Han population as well as the oxygen affinity P50 of the plateau Han and Tibetan populations to determine the basis for different adaptation mechanisms of the Tibetan and Han populations to the plateau environment.
When the plain Han population rapidly entered the plateau, the 2,3-DPG level in the RBCs increased, the oxygen dissociation curve shifted to the right (increased P50), the oxygen affinity of HGB was decreased to increase the oxygen unloading efficiency, and SaO2 was therefore reduced [Citation8]. The results of this study revealed that that on the 3rd day after the Han population of the plains entered the plateau, the P50 value (23.22 mmHg) was slightly higher than that of the population of the plains (22.46 mmHg), and on the 7th day after the Han population of the plains entered the plateau, the P50 value (28.17 mmHg) increased significantly (P < 0.01). This finding suggests that after the healthy Han population rapidly entered the plateau, the HGB oxygen affinity kept decreasing, improving the oxygen release efficiency in the tissue and thus ensuring a normal oxygen supply to the body. After 30 days of altitude acclimatization, the P50 value (17.08 mmHg) of the Han population decreased significantly (P < 0.01), indicating increased HGB oxygen affinity, which helps the lungs bind more oxygen molecules, maintains arterial SaO2, and promotes the plateau adaptation [Citation9]. In conclusion, the RBCs counts and HGB concentration were significantly increased in the early stage after the Han population of the plains entered the plateau and after adaption to the plateau. However, the P50 value first increased as the RBCs counts and HGB concentration increased during the early stage after the Han population of the plains entered the plateau but decreased significantly after adaptation. Therefore, the oxygen unloading efficiency was enhanced during the initial stage to ensure the oxygen supply to the organism, while the oxygen affinity of HGB was increased during the prolonged stay at the plateau, and the arterial SaO2 gradually increased, leading to plateau acclimatization.
Balaban et al. [Citation10] found that the P50 values of the residents of the plains, the plateau-acclimatized population (plain population living at an altitude of 3600–4100 m for 2–3 weeks), and the plateau dwellers (living for generations in the Andean Plateau) were 30.8, 24.8, and 26.5 mmHg, respectively, indicating that the in vivo oxygen dissociation curve of the plateau dwellers was shifted to the left compared to that of the residents of the plains; this change was related to respiratory alkalosis because the three populations had a blood pH of 7.37, 7.46, and 7.43. Consistent with the results of this study, the P50 values of the plateau-acclimatized Han population (17.08 mmHg), the plateau Han population (plateau residence > 10 years) (18.10 mmHg), and the plateau Tibetan population (17.51 mmHg) were significantly lower than that of the Han population of the plains (22.46 mmHg, P < 0.01). The reasons might be as follows: 1. The adaptation in plateau-acclimatized and long-term plateau-dwelling Han populations may be related to changes in the 2,3-DPG level and pH value. 2. The adaptation in plateau Tibetan people may be associated with unique genetic characteristics and evolutionary pressure. The HGB oxygen affinity P50 values of the plateau-acclimatized and long-term plateau-dwelling Han populations are consistent with that of the plateau Tibetan population, and whether genetic factors participate in the HGB oxygen affinity difference should be further studied. Greater oxygen affinity is an important adaptive mechanism in plateau species. In this study, a lower HGB concentrations and P50 values were founded in the Tibetan subjects [Citation11,Citation12]. Thus, it is conceivable that reduced HGB would be associated with a reduction in P50, a trait shown to be associated with the genetic factors in Tibetan [Citation13]. In consistent with Simonson’s research [Citation13], it also showed that there was no correlation between P50 and HGB in Tibetan subjects or when Tibetan and Han Chinese (resistant in plateau at least 2 years) data were analyzed together as a single group. However, whether lower P50 results from genetically based changes in Tibetans remains unclear.
Conclusion
In summary, during the early stage after the Han population of the plains entered the plateau (within 3–7 days after entering the plateau), the RBCs counts, HGB concentration, and P50 values were significantly increased; these changes are a stress compensatory response to maintain the normal tissue oxygen level. After adaptation to the plateau environment for 30 days, the RBCs counts and HGB concentration were maintained at a certain level, and the P50 value was significantly decreased. This change is a feature of the adaptation to the plateau to avoid altitude sickness, such as high-altitude polycythemia and dyspnea. The P50 values of the plateau Han and Tibetan populations were also significantly lower than that of the Han population of the plains, which is a long-term adaptation to the plateau.
Acknowledgments
The authors thank Fengyan Fan and Ying Wang for excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Cuiying Li http://orcid.org/0000-0002-7045-0838
Additional information
Funding
References
- Huerta-Sanchez E, Jin X, Asan X, et al. Altitude adaptation in Tibetans caused by introgression of denisovan-like DNA. Nature. 2014;512(7513):194–197. doi: 10.1038/nature13408
- Petousi N, Croft QP, Cavalleri GL, et al. Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 2014;116(7):893–904.
- Siques P, Brito J, Banegas JR, et al. Blood pressure responses in young adults first exposed to high altitude for 12 months at 3550 m. High Alt Med Biol. 2009;10(4):329–335. doi: 10.1089/ham.2008.1103
- Bigham AW, Lee FS. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev. 2014;28(20):2189–2204. doi: 10.1101/gad.250167.114
- Mangin O. High oxygen affinity hemoglobins. La Revue de Medecine Interne. 2017;38(2):106–112. doi: 10.1016/j.revmed.2016.06.003
- Beall CM, Decker MJ, Brittenham GM, et al. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA. 2002;99(26):17215–17218. doi: 10.1073/pnas.252649199
- Peng Y, Yang Z, Zhang H, et al. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol. 2011;28(2):1075–1081. doi: 10.1093/molbev/msq290
- Wahr JA, Gerber M, Venitz J, et al. Allosteric modification of oxygen delivery by hemoglobin. Anesth Analg. 2001;92(3):615–620. doi: 10.1213/00000539-200103000-00011
- Yalcin O, Cabrales P. Increased hemoglobin O2 affinity protects during acute hypoxia. American J Physiol Heart Circ Physiol. 2012;303(3):H271–H281. doi: 10.1152/ajpheart.00078.2012
- Balaban DY, Duffin J, Preiss D, et al. The in-vivo oxyhaemoglobin dissociation curve at sea level and high altitude. Respir Physiol Neurobiol. 2013;186(1):45–52. doi: 10.1016/j.resp.2012.12.011
- Natarajan C, Inoguchi N, Weber RE, et al. Epistasis among adaptive mutations in deer mouse hemoglobin. Science. 2013;340(6138):1324–1327. doi: 10.1126/science.1236862
- Projecto-Garcia J, Natarajan C, Moriyama H, et al. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc Natl Acad Sci USA. 2013;110(51):20669–20674. doi: 10.1073/pnas.1315456110
- Simonson TS, Wei G, Wagner HE, et al. Increased blood-oxygen binding affinity in Tibetan and Han Chinese residents at 4200 m. Exp Physiol. 2014;99(12):1624–1635. doi: 10.1113/expphysiol.2014.080820