ABSTRACT
Objective: An increasing amount of evidence shows that childhood leukemia is initiated in utero. Birth characteristics initiated in utero, such as gestational age, may play a role in leukemogenesis. The purpose of our meta-analysis is to explore the association between gestational age and childhood leukemia.
Methods: Relevant studies up to 21 April 2017 were collected by searching PubMed and EMBASE databases. Subgroup analysis, sensitivity analysis and publication bias assessment were conducted.
Results: A total of 13 studies were included. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) for preterm birth and postterm birth were 1.06 (0.98, 1.13) and 1.01 (0.90, 1.13) for childhood leukemia, 1.04 (0.97, 1.11) and 1.03 (0.95, 1.12) for acute lymphocytic leukemia (ALL), 1.20 (1.00, 1.44) and 1.20 (1.00, 1.43) for acute myeloid leukemia (AML), compared with full-term birth. Study type and study region were the reasons behind the heterogeneity. In subgroup analyses, the summary ORs with 95% CI for childhood leukemia and ALL were 1.23 (1.07, 1.41) and 1.21 (1.06, 1.39) for postterm birth in cohort studies. No significant changes in sensitivity analyses and no publication bias were observed in our analysis.
Conclusion: Our results suggest that both preterm and postterm infants have an elevated risk of developing AML. In addition, postterm birth increased the risk of childhood leukemia and ALL in cohort studies. However, more studies are warranted to validate these results and explore the biologic mechanisms underlying these relationships.
Introduction
Childhood leukemia has become the most common cancer type of childhood [Citation1], but only a few cases can be explained by known risk factors, such as Down’s syndrome, ionizing radiation and benzene. A growing amount of evidence indicating that childhood leukemia, representing the clonal proliferation of transformed hemopoietic cells driven by mutations, is initiated in utero [Citation2,Citation3]. In this respect, birth characteristics initiated in utero, as birth weight and gestational age, may have important roles in the leukemogenic process. The meta-analysis [Citation4] suggested that high birth weight was a factor that has been implicated in an increased risk of all types of leukemia, while low birth weight enhanced the risk of developing acute myeloid leukemia (AML). In addition, preterm birth infants, who were given birth before 37 completed gestational weeks, are usually born with immature organs and thus are known to be more likely to suffer from respiratory distress syndrome, neurologic morbidities and chronic diseases in their later life [Citation5–7]. Postterm infants, born after 41 completed gestational weeks, are more likely to suffer from abnormal growth, asphyxia and neurologic diseases over their life courses [Citation7,Citation8]. Accordingly, it is interesting to investigate whether gestational age has an influence on the development of childhood leukemia and its subtypes, acute lymphocytic leukemia (ALL) and AML.
Huang et al. [Citation9] published a meta-analysis in 2016 that indicated preterm birth was related to a higher risk of acute childhood leukemia and AML. Nevertheless, no significant association was found for ALL. At the same time, no data analysis was performed for the relationships among postterm birth and childhood leukemia, ALL and AML. Interestingly, different results regarding the association between childhood leukemia risk and postterm birth were reported. For example, some epidemiologic studies suggested a positive association between postterm birth and increased risk of ALL [Citation10–14]; however, other studies reported that there was no relation [Citation15–19]. Importantly, this discrepancy might be largely due to the small sample size, different study populations and study designs of each single study. Therefore, we updated the published meta-analysis, utilizing all available evidence to date, and summarized the potential effect of various gestational age groups on the probability of developing childhood leukemia and its subtypes.
Methods
Our meta-analysis followed the guidance provided in the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [Citation20].
Search strategy
The PubMed and EMBASE databases were searched with the keywords listed below through to 21 April 2017. The keywords employed were (birth characteristics OR perinatal characteristics OR preterm OR postterm OR gestational OR birth weight) AND (infant OR child OR childhood) AND (leukemia OR malignancy OR cancer). All existing articles in English were considered. Furthermore, we looked through the additional references identified through the citations of the correlative articles and reviews.
Selection criteria
The potential studies were chosen according to the following criteria: (1) a case–control or cohort study; (2) the cases were diagnosed with childhood leukemia, ALL and/or AML; (3) preterm, full-term or postterm gestational age were presented; (4) the number of controls and cases in different gestational ages was reported; (5) studies with children who had any diseases that increased the risk of leukemia were eliminated. When multiple studies were found to have been conducted on either an overlapping or the same population, the most informative research was included in our study. The literature review was conducted by two authors (Yang-Feng Wang and Yi-Ni Liu).
Data extraction and quality assessment
Two authors (Yang-Feng Wang and Li-Qun Wu) evaluated the included studies blindly and independently. All disagreements were settled through consensus. The Newcastle-Ottawa-Scale (NOS) was utilized to assess the quality of the included papers. The scores ranged from 0 to 9, and studies with NOS scores of 7–9, 4–6 and 0–3 were defined as high, moderate and low quality, respectively.
Statistical analysis
Stata 12.0 software was used for our research. Results were considered statistically significant when a two-sided P was under 0.05. Childhood leukemia cases were a combination of both ALL and AML cases when they were not reported unambiguously. To report the connection between gestational age and childhood leukemia and its subgroups, the number of controls and cases were used to calculate the unadjusted odds ratios (ORs) with 95 percent confidence intervals (95% CIs) regarding diverse gestational age. To our knowledge, the strength of causal generalization of cohort studies is better than case–control studies, and variables can only be converted from high to low which cannot do the opposite direction of the conversion from Applied Methodology for Evidence-Based Medicine. OR was used as the common measure of association in this study, reflecting relative risk (RR). The heterogeneity between the studies was evaluated by I2 and the Q-statistic test. If heterogeneity existed (I2 > 50% and/or P < 0.1), the random-effect model was used; if not, the fixed-effect model was used. We performed subgroup analyses to identify the origin of heterogeneity. Egger’s and Begg’s tests were performed to evaluate the publication bias. Sensitivity analyses were conducted to assess the stability of the results.
Results
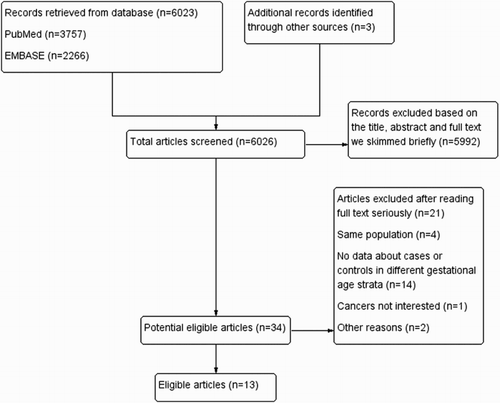
The detailed results of the retrieval strategy and research inclusion procedure are summarized in . Thirteen eligible studies were included [Citation10–19,Citation21–23]. The primary features of the eligible studies are presented in . Among the included studies, six studies were from North America; one study was from both North America and Europe; six studies were from Europe. This analysis consisted of 12,944 cases and 3,613,706 controls from 8 studies about childhood leukemia, 15,039 cases and 4,054,907 controls from 11 studies about ALL, and 1,720 cases and 3,593,462 controls from six studies about AML. Except for three cohort studies, all were case–control studies. Preterm birth was defined as <37 weeks or ≤37 weeks in all studies except one. Postterm birth was mostly defined as ≥41 weeks or ≥42 weeks.
Table 1. Characteristics of studies on the association between gestational age and risk of leukemia.
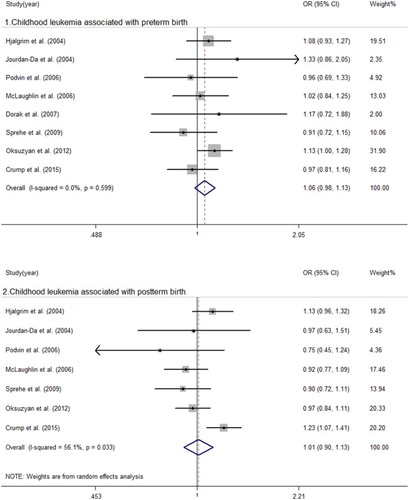
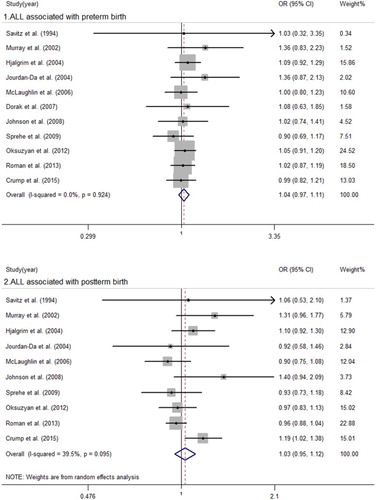
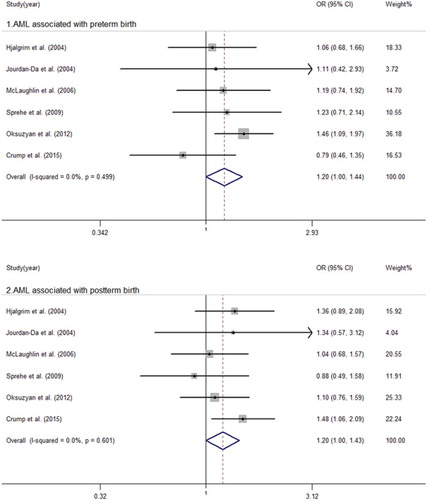
The main analysis is summarized in and –. We obtained that there was significant heterogeneity between childhood leukemia, ALL and postterm birth with I2 = 56.1 (P = 0.033), I2 = 39.5 (P = 0.095), respectively. We then built a random-effect model to evaluate the summary ORs. There were no significant associations between postterm birth and childhood leukemia (OR = 1.01, 95% CI = 0.90–1.13) or ALL (OR = 1.03, 95% CI = 0.95–1.12). We used a fixed-effect model to calculate the remaining pooled ORs. The results suggested an increased risk of AML in those who were postterm birth infants (OR = 1.20, 95% CI = 1.00–1.43) and preterm birth infants (OR = 1.20, 95%CI = 1.00–1.44) when compared to full-term births. No significant associations were observed between preterm birth and childhood leukemia (OR = 1.06, 95% CI = 0.98–1.13) or ALL (OR = 1.04, 95% CI = 0.97–1.11).
Table 2. Pooled association between gestational age and childhood leukemia.
To discover the origin of heterogeneity, subgroup analyses are shown in . Consistent results were observed across sample size, child’s age of leukemia diagnosis and postterm gestational age. Subgroup analyses indicated that study type and study region contributed to the heterogeneity. In cohort studies, the summary ORs with 95% CIs for postterm birth associated with childhood leukemia and ALL were 1.23 (1.07, 1.41) and 1.21 (1.06, 1.39). In Europe, the pooled ORs with 95% CIs for postterm birth associated with childhood leukemia and ALL were 1.17 (1.06, 1.30) and 1.15 (1.04, 1.28). In the sensitivity analyses, no significant changes were observed after excluding the original study that possibly influenced the pooled results. No publication bias was observed, as shown in .
Table 3. Subgroup analyses of the association between postterm age and childhood leukemia, acute lymphoblastic leukemia (ALL).
Discussion
This analysis demonstrated modest borderline associations between postterm delivery, preterm birth and AML. Subgroup analyses by study types further showed postterm birth increased the risk of childhood leukemia and ALL in cohort studies. A previous manuscript published by Huang et al. [Citation9] found that preterm delivery was associated with increased risk of acute childhood leukemia and AML; there was no significant relationship between preterm birth and ALL. The same trend with different results were reported in our article, mainly because we brought into different studies that relied on a diverse search strategy and inclusion criteria. In addition, the analysis reported by Huang et al. [Citation9] did not examine postterm delivery. We added six new studies [Citation11,Citation13,Citation14,Citation17,Citation19,Citation23] and investigated the relationships among postterm delivery and childhood leukemia, ALL and AML. Additionally, our meta-analysis is the first to report that postterm birth has modestly increased relationship to AML risk.
We found that study type and study region were the sources of heterogeneity: the various epidemiologic studies had different design ideas, and the two cohort studies had larger sample sizes than the case–control studies. In addition, gestational age was affected by ethnic variation that developed evolutionarily different mechanisms in gestational duration [Citation24], especially for premature infants [Citation25]. In subgroup analyses, postterm birth increased the risk of childhood leukemia and ALL in cohort studies. Postterm birth did not truly raise the risk of childhood leukemia or ALL in Europe, mainly due to the influence of study type. When the two cohort studies that investigated the European population were eliminated, the pooled ORs with 95% CIs for childhood leukemia and ALL associated with postterm birth were 1.11 (0.95, 1.29) and 1.07 (0.91, 1.26).
The underlying mechanisms for the association between gestational age and AML still are not well-established. Some studies indicated that the insulin-like growth factor (IGF) system, mainly IGF-1 and IGF-2, involved in the regulation of cell proliferation, differentiation, and apoptosis [Citation26,Citation27], was the most important determinants of the relationships among fetal growth and leukemia, ALL and AML [Citation4,Citation9,Citation28,Citation29]. Moreover, the expression levels of IGF-1, IGF-2 and insulin-like growth factor binding protein (IGFBP)-3 were found to increase with gestational age, while that of IGFBP-2 was observed to decrease in fetal blood [Citation30–32]. Some premature infants experienced postnatal catch-up growth that stimulated the expression of IGF [Citation33,Citation34]. Postterm pregnancies were associated with an approximately two-fold increased risk of macrosomia [Citation35], and high birth weight was shown to be related to an increased risk of AML [Citation4]. IGFBP-2, overexpressed in multiple tumor types [Citation36,Citation37], was observed to be widely expressed during fetal development and is a major binding protein in the uterus [Citation30,Citation38]. In addition, patients with AML showed higher IGF-2 and IGFBP-2 expression accompanied by lower IGF-1 and IGFBP-3 levels in serum [Citation39–41]. In summary, the underlying mechanisms may be partially dependent on the concept that IGFBP-2 is mainly expressed in prematurity and IGF-2 maintains higher expression levels in postterm delivery.
Our meta-analysis may have the following limitations. First, although most of our data were obtained from birth records and cancer registry, a number of individuals had missing data on some variables. Second, we had no access to sufficient data to examine the association between gestational age and ALL subtypes. Third, many potential confounders should be considered, as gender, birth weight, age at diagnosis and maternal age. However, we could not calculate the adjusted summary ORs relied on both unadjusted [Citation13,Citation17,Citation19,Citation22] and adjusted [Citation12,Citation15,Citation16,Citation18] analysis models. Finally, we could not evaluate the dose–response relationship between gestational age and leukemia, because it was difficult to accurately assess the gestational age since the lower and higher bounds of gestational age were not specified in all studies. Despite these limitations, there are some strengths in our research. First, all of the included studies had a moderate or high NOS score. Second, no publication bias was observed, and the results of our study were stable. Finally, a large number of cases and controls were contained in our study.
In conclusion, we conducted the meta-analysis to examine the relationships among different gestational ages and the risk of childhood leukemia and its subgroups. Preterm birth and postterm birth were associated with a modestly increased risk of AML. In addition, postterm birth increased the risk of childhood leukemia and ALL in cohort studies. Further studies are needed to explore the association of gestational age with childhood leukemia, ALL and AML and explore the biologic mechanisms underlying these associations.
Acknowledgments
The authors extend a sincere acknowledgement to all members in Professor H. Wang’s laboratory for their precious assistances and comments of the analysis.
Disclosure statement
The authors declare that they have no potential conflict of interest.
Notes on contributors
Yang-Feng Wang, the first author of the article, Li-Qun Wu and Yi-Ni Liu are postgraduate students. Yong-yi Bi who has a PhD degree is a professor. Hong Wang who also has a PhD degree is an associate professor.
ORCID
Yang-Feng Wang http://orcid.org/0000-0001-6558-2169
Li-Qun Wu http://orcid.org/0000-0002-3270-960X
Yi-Ni Liu http://orcid.org/0000-0003-0170-1645
Yong-Yi Bi http://orcid.org/0000-0001-6221-3341
Hong Wang http://orcid.org/0000-0001-7540-5254
Additional information
Funding
References
- Linet MS, Brown LM, Mbulaiteye SM, et al. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0–19 years. Int J Cancer. 2016;138:1862–1874. doi: 10.1002/ijc.29924
- Wiemels JL. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99:3801–3805. doi: 10.1182/blood.V99.10.3801
- Wiemels JL, Cazzaniga G, Daniotti M, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–1503. doi: 10.1016/S0140-6736(99)09403-9
- Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1
- Savitz DA, Werner EF. Invited commentary: isolating preterm birth to assess its impact. Am J Epidemiol. 2015;182:759–761. doi: 10.1093/aje/kwv166
- Seikku L, Gissler M, Andersson S, et al. Asphyxia, neurologic morbidity, and perinatal mortality in early-term and postterm birth. Pediatrics. 2016;137:e20153334–e20153334. doi: 10.1542/peds.2015-3334
- Ehrenstein V, Pedersen L, Holsteen V, et al. Postterm delivery and risk for epilepsy in childhood. Pediatrics. 2007;119:e554–e561. doi: 10.1542/peds.2006-1308
- Huang Q, Gao Y, Zhong M, et al. Preterm birth and subsequent risk of acute childhood leukemia: a meta-analysis of observational studies. Cell Physiol Biochem. 2016;39:1229–1238. doi: 10.1159/000447828
- Savitz DA, Ananth CV. Birth characteristics of childhood cancer cases, controls, and their siblings. Pediatr Hematol Oncol. 1994;11:587–599. doi: 10.3109/08880019409141806
- Murray L, McCarron P, Bailie K, et al. Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer. 2002;86:356–361. doi: 10.1038/sj.bjc.6600012
- Hjalgrim LL, Rostgaard K, Hjalgrim H, et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. JNCI. 2004;96:1549–1556. doi: 10.1093/jnci/djh287
- Johnson KJ, Soler JT, Puumala SE, et al. Parental and infant characteristics and childhood leukemia in Minnesota. BMC Pediatr. 2008;8:123. doi: 10.1186/1471-2431-8-7
- Crump C, Sundquist J, Sieh W, et al. Perinatal and familial risk factors for acute lymphoblastic leukemia in a Swedish national cohort. Cancer. 2015;121:1040–1047. doi: 10.1002/cncr.29172
- Jourdan-Da Silva N, Perel Y, Méchinaud F, et al. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br J Cancer. 2004;90:139–145. doi: 10.1038/sj.bjc.6601384
- McLaughlin CC, Baptiste MS, Schymura MJ, et al. Birth weight, maternal weight and childhood leukaemia. Br J Cancer. 2006;94:1738–1744. doi: 10.1038/sj.bjc.6603173
- Sprehe MR, Barahmani N, Cao Y, et al. Comparison of birth weight corrected for gestational age and birth weight alone in prediction of development of childhood leukemia and central nervous system tumors. Pediatr Blood Cancer. 2009;13:n/a–n/a. doi: 10.1002/pbc.22308
- Oksuzyan S, Crespi CM, Cockburn M, et al. Birth weight and other perinatal characteristics and childhood leukemia in California. Cancer Epidemiol. 2012;36:e359–e365. doi: 10.1016/j.canep.2012.08.002
- Roman E, Lightfoot T, Smith AG, et al. Childhood acute lymphoblastic leukaemia and birthweight: insights from a pooled analysis of case–control data from Germany, the United Kingdom and the United States. Eur J Cancer. 2013;49:1437–1447. doi: 10.1016/j.ejca.2012.11.017
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008
- Podvin D, Kuehn CM, Mueller BA, et al. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. 2006;20:312–322. doi: 10.1111/j.1365-3016.2006.00731.x
- Dorak MT, Pearce MS, Hammal DM, et al. Examination of gender effect in birth weight and miscarriage associations with childhood cancer (United Kingdom). Cancer Cause Control. 2007;18:219–228. doi: 10.1007/s10552-006-0093-8
- Crump C, Sundquist J, Sieh W, et al. Perinatal risk factors for acute myeloid leukemia. Eur J Epidemiol. 2015;30:1277–1285. doi: 10.1007/s10654-015-0063-0
- Patel RR. Does gestation vary by ethnic group? A London-based study of over 122 000 pregnancies with spontaneous onset of labour. Int J Epidemiol. 2004;33:107–113. doi: 10.1093/ije/dyg238
- Pennell CE, Jacobsson B, Williams SM, et al. Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol. 2007;196:107–118. doi: 10.1016/j.ajog.2006.03.109
- Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J
- Dupont J, Pierre A, Froment P, et al. The insulin-like growth factor axis in cell cycle progression. Horm Metab Res. 2003;35:740–750. doi: 10.1055/s-2004-814162
- Milne E, Greenop KR, Metayer C, et al. Fetal growth and childhood acute lymphoblastic leukemia: findings from the childhood leukemia international consortium. Int J Cancer. 2013;124.
- Hjalgrim LL. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158:724–735. doi: 10.1093/aje/kwg210
- Langford K, Nicolaides K, Miell JP. Maternal and fetal insulin-like growth factors and their binding proteins in the second and third trimesters of human pregnancy. Hum Reprod. 1998;13:1389–1393. doi: 10.1093/humrep/13.5.1389
- Smerieri A, Petraroli M, Ziveri MA, et al. Effects of cord serum insulin, IGF-II, IGFBP-2, IL-6 and cortisol concentrations on human birth weight and length: pilot study. Plos One. 2011;6:e29562. doi: 10.1371/journal.pone.0029562
- Lauszus FF, Fuglsang J. IGF-1 is associated with fetal growth and preterm delivery in type 1 diabetic pregnancy. Gynecol Endocrinol. 2016;32:488–491. doi: 10.3109/09513590.2015.1134477
- Stawerska R, Szałapska M, Hilczer M, et al. Ghrelin, insulin-like growth factor I and adipocytokines concentrations in born small for gestational age prepubertal children after the catch-up growth. J Pediatr Endocrinol Metab. 2016;29:733–945. doi: 10.1515/jpem-2015-0463
- Miles HL, Derraik JGB, Chiavaroli V, et al. Response to IGF-1 generation test in short prepubertal children born very preterm or at term. Horm Res Paediatr. 2015;84:298–304. doi: 10.1159/000439233
- Wang M, Fontaine P. Common questions about late-term and postterm pregnancy. Am Fam Physician. 2014;90:160–165.
- Elminger MW, Bell M, Schüett BS, et al. Transactivation of the IGFBP-2 promoter in human tumor cell lines. Mol Cell Endocrinol. 2001;175:211–218. doi: 10.1016/S0303-7207(00)00454-8
- Menouny M, Binoux M, Babajko S. IGFBP-2 expression in a human cell line is associated with increased IGFBP-3 proteolysis, decreased IGFBP-1 expression and increased tumorigenicity. Int J Cancer. 1998;77:874–879. doi: 10.1002/(SICI)1097-0215(19980911)77:6<874::AID-IJC13>3.0.CO;2-1
- Ko JM, Park HK, Yang S, et al. Influence of catch-up growth on IGFBP-2 levels and association between IGFBP-2 and cardiovascular risk factors in Korean children born SGA. Endocr J. 2012;59:725–733. doi: 10.1507/endocrj.EJ12-0080
- Dawczynski K, Steinbach D, Wittig S, et al. Expression of components of the IGF axis in childhood acute myelogenous leukemia. Pediatr Blood Cancer. 2008;50:24–28. doi: 10.1002/pbc.21294
- Dawczynski K, Kauf E, Zintl F. Changes of serum growth factors (IGF-I,-II and IGFBP-2,-3) prior to and after stem cell transplantation in children with acute leukemia. Bone Marrow Transplant. 2003;32:411–415. doi: 10.1038/sj.bmt.1704149
- Wu HK, Weksberg R, Minden MD, et al. Loss of imprinting of human insulin-like growth factor II gene, IGF2, in acute myeloid leukemia. Biochem Biophys Res Commun. 1997;231:466–472. doi: 10.1006/bbrc.1997.6127