ABSTRACT
Multipotent mesenchymal stromal cells (MSCs) play a central role within the bone marrow (BM) niche, supporting hematopoiesis via soluble factors like cytokines and chemokines. In our study, we sought to investigate the effect of blocking transforming growth factor beta 1 (TGF-β1) and C-X-C motif chemokine 12 (CXCL12) receptor CXCR4 on acute myeloid leukemia (AML) cells in an MSC co-culture system.
Human MSCs were obtained by BM aspirates and their phenotype and functional properties were confirmed in vitro. Co-cultures of AML cells on MSCs were initiated and compared to those on mouse fibroblasts (MS-5) and liquid cultures. Additionally, the effect of blocking CXCR4 and TGF-β1 on AML cells was tested with and without the addition of cytarabine.
MSCs from BM showed a typical phenotype and differentiation pattern. Co-culture of AML cells on MSCs resulted in a significantly higher proliferation capacity than on MS-5 or liquid culture. Blockade of TGF-β1 increased AML cell proliferation and chemosensibility, while the CXCR4 antagonist plerixafor showed anti-proliferative effects and did not change cytarabine-induced cell death compared to control.
Human MSCs are potent feeder cells, able to maintain AML cells in long-term culture. This favorable co-existence seems to be due in part to molecules important for communication within the niche. Blockade of TGF-β1 and CXCL12 was associated with different effects on AML cell proliferation and chemotherapy resistance.
These findings suggest a strong supporting affinity between MSCs and AML cells within the leukemic niche, where TGF-β1 and CXCL12 pathways play an important role.
Introduction
Hematopoietic stem cells (HSC) reside in the bone marrow (BM) within a specialized microenvironment (niche) that is crucial for their function and maintenance. This niche is comprised of a variety of cellular and extracellular components [Citation1] including multipotent mesenchymal stromal cells (MSCs), first demonstrated in the non-hematopoietic system of the BM [Citation2] almost 50 years ago. In 2006, the International Society for Cellular Therapy (ISCT) issued the minimal criteria for defining MSCs, requiring adherence to plastic under standard culture conditions, the expression of defined surface molecules and the ability to differentiate into the osteogenic, chondrogenic and adipogenic lineages [Citation3]. MSCs were identified as key regulatory components of HSC within the BM niche as they are located perivascularly adjacent to HSC and express HSC maintenance related genes at a high level [Citation4].
The HSC-niche interaction is believed to be in great part regulated by cytokines secreted either by stromal cells or hematopoietic cells. TGF-β1 and C-X-C motif chemokine 12 (CXCL12) secreted by MSCs have been demonstrated to be key factors within the BM niche governing hematopoietic cell proliferation and survival [Citation5–9]. Moreover, these cytokines are involved in maintaining HSC in the BM: TGF-β1 by keeping HSC quiescent, CXCL12 by promoting HSC homing and anchoring in the niche through its receptor CXCR4 [Citation1].
Similar mechanisms were described in the interaction between MSCs and acute myeloid leukemia (AML) cells, hence impairing the cytotoxic effects of chemotherapy regimens [Citation5,Citation6,Citation10–12]. Therefore, blockade of TGF-β1 or the CXCL12/CXCR4-axis could supposedly enhance chemotherapy sensibility of AML cells. Recently, it was shown that TGF-β1 and CXCL12 pathways are interconnected by Smad2/3 signaling molecules [Citation13,Citation14], strengthening the conception to investigate both cytokines simultaneously.
In this study we designed co-culture experiments using human MSCs as feeder layer mimicking the physiological niche to investigate the effect of transforming growth factor beta 1 (TGF-β1) and CXCR4 inhibition on proliferation and survival of AML cells. For this, a neutralizing mouse anti-human TGF-β1 antibody and a commercially available CXCR4 antagonist (plerixafor) were used as blocking agents, and the impact on AML cells was analyzed also in the presence of the major chemotherapeutic drug cytarabine.
Material and methods
MS-5 cell line
The murine stromal cell line MS-5 was obtained from DSMZ (German Collection of Microorganisms and Cell Cultures) Leibniz Institute (Braunschweig, Germany) and cultured in DMEM (Life Technologies, Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS, PAA Laboratories, Pasching, Austria), 100 U/ml penicillin-100 µg/ml streptomycin (Sigma-Aldrich, Darmstadt, Germany), 2 mM L-glutamine (Sigma-Aldrich), 10 mM HEPES (Sigma-Aldrich), 1 mM MEM sodium pyruvate, 1% MEM vitamins solution and 50 µM 2-mercaptoethanol (Gibco Invitrogen, Darmstadt, Germany) at 37°C and 5% CO2. The cells were routinely subcultured by trypsinization using a trypsin (0.05%)-EDTA (0.02%) solution (Sigma-Aldrich).
Patient characteristics
BM aspirates were obtained from the iliac crest of 10 AML patients after written informed consent. The procedures are in accordance with the current version of the Helsinki Declaration and were approved by the local Ethics Committee of Human Experimentation, University Regensburg. AML patient and disease characteristics are summarized in .
Table 1. Patients’ characteristics.
Mesenchymal stromal cells
Expansion
Human BM aspirate from healthy donors (ethical approval 11-101-0303, Ethical Committee of the University Hospital of Regensburg) was diluted with DMEM supplemented with 10% FBS and 2.5 mg/ml gentamicin (Life Technologies, Darmstadt, Germany) and plated in 100 mm cell culture dishes (BD Falcon, Heidelberg, Germany). Medium was changed twice weekly and cell growth and morphology were regularly checked and documented using a Hund Willovert S microscope (Hund Wetzlar, Wetzlar, Germany). When confluence was reached, MSCs were trypsinized and further expanded in 75 cm2 cell culture flasks (Corning Costar, Bodenheim, Germany). After 2–4 weeks (70–90% confluence), MSCs were harvested and phenotypically and functionally analyzed. The specific MSC phenotype was determined by flow cytometric analysis of cell surface proteins as described below.
Differentiation
For functional characterization, MSCs (1.9 or 3.8 × 104 cells per well) were plated in 12-well plates and differentiation into osteoblasts, chondrocytes and adipocytes was initiated using specific differentiation media (StemPro Osteogenesis Differentiation Kit, StemPro Chondrogenesis Differentiation Kit and StemPro Adipogenesis Differentiation Kit, respectively; Gibco Invitrogen) supplemented with gentamycin. After 14–21 days, cells were fixed with 4% formaldehyde (Carl Roth, Karlsruhe, Germany) solution for 30 min and washed with PBS (Sigma-Aldrich) or dH2O. To assess osteogenic, chondrogenic and adipogenic differentiation, cells were stained with silver nitrate solution (3%, Von Kossa Calcium Kit, Polysciences, Eppelheim, Germany), Safranin-O solution (0.5% in dH2O, Morphisto, Frankfurt, Germany) and Oil Red O solution (0.5% in propylene glycol; Sigma-Aldrich) for 30–60 min, respectively and visualized using the microscope Axiovert 200 M MAT and AxioVision Rel. 4.8 software (Carl Zeiss GmbH, Jena, Germany). Only MSC samples displaying a characteristic phenotype and differentiation pattern were used for further experiments and stored in liquid nitrogen until use.
AML cells
Samples from patients were obtained from BM aspirates at first admission to our department (ethical approval 051097, ethical committee of the University Hospital of Regensburg). Cells were then enriched for mononuclear cells (MNCs) by Ficoll Hypaque (GE Healthcare Bio-Sciences, Pasching, Austria) density centrifugation and cryopreserved in liquid nitrogen until use. The proportion of CD34+ AML cells was 80–90%.
Liquid culture and analysis of MS-5 or MSC feeder layer on AML cell proliferation support
For liquid culture experiments, 2 × 106 AML cells (MNC, AML samples 1–4) were cultured for 7 days in multiwell cell culture plates (20 wells á 105 AML cells) containing RPMI 1640 medium supplemented with 100 U/ml penicillin-100 µg/ml streptomycin (Sigma-Aldrich), 2 mM L-glutamine (Sigma-Aldrich), 1% vitamins solution, 1% MEM non-essential amino acid solution, 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol (Gibco Invitrogen) and 10% FBS (PAA Laboratories).
For in vitro long-term maintenance of AML cells, co-culture experiments using either murine fibroblasts (MS-5) or human MSC as feeder layer were performed. In brief, MS-5 cells were plated in gelatine (0.1%, PAN Biotech, Aidenbach, Germany)-coated 24-well plates at a density of 7.2 × 104 cells per well 24 hours prior to the experiment. MSCs at passage 2 were seeded at 3.8 × 104/well one week before the start of the co-culture. AML cells (6 × 105 MNC) were resuspended in Iscove’s-modified Dulbecco’s Medium (IMDM, Life Technologies) as described below (section ‘Analysis of blocking TGF-β1 and CXCR4 on AML cells’) and cultured on MS-5 or MSC up to 12 weeks with weekly half-medium changes. Cell morphology was routinely monitored using an inverted microscope and photographed (Axiovert 200 M MAT microscope). At the end of the culture period, cells were harvested, stained with the fluorochrome-conjugated monoclonal mouse anti-human antibody (mAb) CD45-PE (clone HI30, BD Pharmingen, Franklin Lakes, NJ, USA) and the amount of hematopoietic cells was determined using an LSR II flow cytometer (BD).
Analysis of blocking TGF-β1 and CXCR4 on AML cells
For evaluating the effect of blocking TGF-β1 and CXCR4 on AML blast chemotherapy sensitivity, co-culture experiments using human MSC as feeder layer were performed. Frozen AML samples were thawed and labeled for subsequent cell sorting of the CD34+ and, to consider CD34+ clones with different stages of maturity, in some experiments also the CD38+ and CD38− fraction, respectively. For this, cells were stained for 30 min at 4°C with combinations of anti-CD45-FITC (clone HI30, BD Pharmingen), anti-CD34-APC (clone 581, BioLegend, San Diego, CA, USA) and anti-CD38-PE (clone HIT2, BioLegend) mAbs. After washing with PBS/2% FBS, propidium iodide (PI, 0.5 µg/ml, Sigma-Aldrich) was added to allow discrimination of dead cells. Cell sorting was performed using a Becton Dickinson FACSAria IIu cell sorter (BD Biosciences, Heidelberg, Germany). CD34+CD38+ and CD34+CD38− AML cell subpopulations were then resuspended in IMDM supplemented with 12.5% FBS, 12.5% horse serum (PAA Laboratories), 100 U/ml penicillin-100 µg/ml streptomycin (Sigma-Aldrich), 2 mM L-glutamine (Sigma-Aldrich), 1 µM hydrocortisone (Rotexmedica, Trittau, Germany), 57.2 µM 2-mercaptoethanol (Life Technologies), 20 ng/ml IL-3, 20 ng/ml TPO and 20 ng/ml G-CSF (all from PeproTech, Rocky Hill, NY, USA) and 1–5 × 105 cells were cultured on MSC for 1–2 weeks with weekly half-medium changes. To investigate the effect of TGF-β1 and CXCR4 inhibition on proliferation and apoptosis of AML cells a neutralizing mouse anti-human TGF-β1 antibody (IgG1, clone 9016, R&D Systems, Minneapolis, MN, USA) and plerixafor (AMD3100, Biomol, Hamburg, Germany) was used, respectively, and added daily to MSC-AML co-culture. All drugs used were dissolved in DMSO and consequently DMSO was added as a control in order to normalize treatment effects. To determine the chemotherapy sensitivity of leukemic cells, they (AML samples 8–10) were either treated with or without anti-TGF-β1 (0.5 µg/ml) and plerixafor (11 µg/ml) and additionally with cytarabine (0.5 μM, Cell Pharm, Bad Vilbel, Germany) 24 hours before analysis. After 7 and 14 days, proliferation, cell cycle progression and apoptosis of CD34+CD38+ and CD34+CD38− AML cells were measured by flow cytometry as described below.
Flow cytometry
Analysis of MSC surface markers
To confirm the specific MSC phenotype cells were harvested and stained for 30 min at 4°C with anti-CD29-Alexa Flour 488 (clone TS2/16), anti-CD44-PE (clone BJ18), anti-CD73-APC (clone AD2), anti-CD90-PE (clone 5E10), anti-CD105-Alexa Flour 488 (clone 43A3) mAbs and the corresponding isotype control antibodies. Hematopoietic cells were excluded by CD45 and CD34 staining (CD45-PerCP, clone HI30 and CD34-APC, clone 581; all Abs were obtained from BioLegend, San Diego, CA, USA). Analysis was performed using a Becton Dickinson FACSCalibur flow cytometer (BD, East Rutherford, NJ, USA).
Analysis of AML cell proliferation
Effects of anti-TGF-β1 and plerixafor +/− cytarabine on AML cell proliferation were assessed with the fluorescent dye carboxyfluorescein diacetate succinimydil ester (CFSE, Molecular Probes, Eugene OR, USA). CFSE binds covalently to intracellular molecules and fluorescence dilution after each cell division can be analyzed. For this, 5 × 106 sorted CD34+CD38+ or CD34+CD38− AML cells were stained with CFSE (0.5 µM in PBS) for 10 min at 37°C in the dark. Subsequently, ice-cold PBS/20% FBS was added and after washing, cells were co-cultured with MSC in a 24-well plate. After one week, cells were harvested, stained with anti-CD73-APC and sorted (BD FACSAria IIu) for the CD73− fraction to exclude MSC from the analysis. CFSE fluorescence of CD45+CD73− AML cells was then determined by flow cytometry (BD FACSCalibur) and the division history was calculated.
Analysis of cell cycle
Cell cycle of AML cells was determined by PI staining of DNA. After one week of co-culture with MSC +/− anti-TGF-β1, plerixafor +/− cytarabine, cells were harvested and sorted for the CD73− cell fraction. Subsequently, cells were washed and resuspended in PBS (1 × 106 in 200 µl). Then, ice-cold ethanol (70%, in dH2O) was slowly added and cell suspension was incubated for 4 hours on ice. After centrifugation, cells were incubated with PBS containing 25 µg/ml PI and 100 µg/ml RNAse (Applichem, Darmstadt, Germany) at 37°C. Measurement of DNA content was then performed on a FACSCalibur (BD). The percentages of cells within the S and G2 phase were added up for the CD34+/CD38− as well as for the CD34+/CD38+compartments.
Analysis of apoptosis
Apoptosis of AML cells was determined after 2 weeks of MSC co-culture with or without anti-TGF-β1, plerixafor +/− cytarabine. Cells were harvested and stained with mouse anti-human CD45-Pacific Orange (clone HI30, Invitrogen, Carlsbad, CA, USA), CD34-PE (clone 581, BioLegend), CD38-Pacific Blue (clone HIT2, EXBIO, Praha, Czech Republic), CD73-APC (BioLegend) and CD117-PE-Cy7 (clone 104D2, BioLegend) for 30 min at 4°C. After washing twice with cold PBS, cells were further processed using the FITC Annexin V Apoptosis Detection Kit (BD, Frankin Lakes, NJ, USA) according to manufacturer’s instruction. Annexin V+PI− apoptotic cell populations were analyzed using a BD LSR II flow cytometer.
Statistical analysis
Data are presented as average +/− standard error of the mean (SEM). Student’s t-test, one-way or two-way analysis of variance (ANOVA) was used for determining statistical significance, a p-value <0.05 was considered statistically significant. Analyses were performed using PRISM 7 (Graphpad, San Diego, CA, USA) statistical software. FlowJo (Ashland, OR, USA) was used to analyze flow cytometric data.
Results
Phenotype and differentiation pattern of human primary MSC
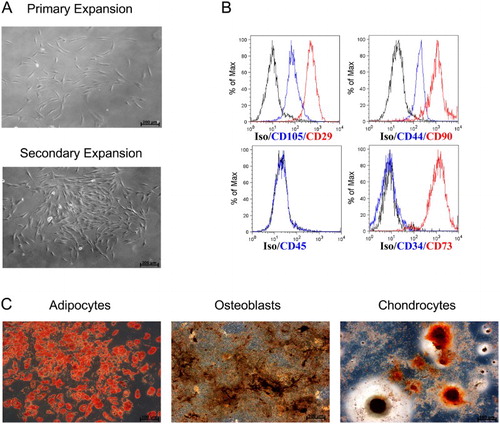
In a first step, successful generation of MSC with well-defined quality parameters was proved by morphological, phenotypical and functional analyses for all MSC used. As shown exemplary in (A), human MSC isolated from the BM of one healthy donor showed characteristic spindle-shaped morphology and plastic adherence when expanded in culture dishes (primary expansion) and cell culture flasks (secondary expansion). Further, the MSC specific phenotype was determined by flow cytometry, i.e. the expression of CD29, CD44, CD73, CD90, CD105 and the lack of CD45 and CD34 expression ((B)). Functional characterization of the MSC was performed by proving their differentiation ability to adipocytes (red), osteoblasts (black) and chondrocytes (red/orange) with the use of standard staining methods ((C)). Regarding the surface marker expression and differentiation capability of MSC from different donors, we could not observe outliers supporting the reproducibility of our harvest and expansion method. None of the MSC samples ever failed to differentiate into the three stromal cell lines.
Figure 1. Characterization of human MSC from BM aspirates. (A) Representative microscopic pictures (100×) of the primary expansion culture of MSC in a 100 mm cell culture dish and secondary expansion culture in a 75 cm2 flask. (B) Flow cytometric analysis of the surface proteins CD29, CD34, CD44, CD45, CD73, CD90 and CD105 on MSC. (C) In vitro differentiation ability of MSC: Oil Red stain for adipogenesis, Von Kossa stain for osteogenesis, Safranin O stain for chondrogenesis (microscopic images 100×).
Human primary MSC are superior in maintaining AML compared to murine MS-5 stromal cells or liquid culture
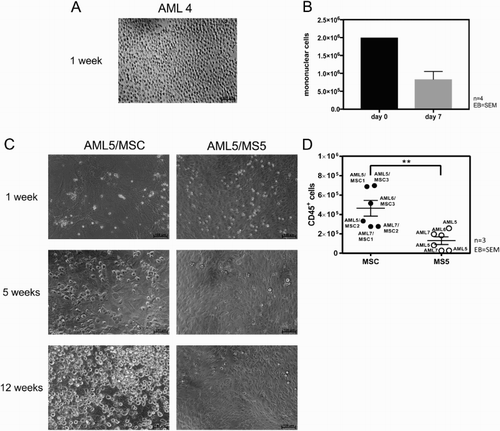
Leukemic cells are difficult to maintain in the absence of a non-hematopoietic feeder layer (15). This is consistent with our own findings, showing a 2.6-fold decrease of AML cells (AML samples 1–4) after 7 days when cultured in RPMI alone ((A,B)). Previously, the murine stromal cell line MS-5 [Citation15] and human BM-MSC have been used to expand primary human AML cells [Citation16]. We now investigated which of these two feeder cells are more effective in maintaining AML cells in culture. As shown in (C), primary AML cells can be maintained up to 12 weeks on MSC, while in the co-culture on MS-5 cells significantly less leukemic cells were maintained. In accordance to the morphology, flow cytometry analysis demonstrated a significant higher viable absolute CD45+ cell count ((D)) after 12 weeks for MSC cultured AML cells as compared to MS-5 cultured AML cells (4.64 × 105 ± 8.06 × 104 vs. 1.29 × 105 ± 3.95 × 104, p = 0.004).
Figure 2. Human MSCs are more effective than murine MS-5 stromal cell line in maintaining AML cells. (A) Representative light microscopic picture (100×) of AML cells cultured for 7 days in liquid culture without feeder cells. (B) Absolute numbers of MNCs from samples AML1-4 after 7 days of liquid culture (n = 4, error bar = SEM). (C) Representative microscopic images (100×) of AML cells co-cultured for 12 weeks on MSC and MS-5 stromal cell line, respectively. (D) Absolute number of viable CD45+ AML cells from three patients, harvested after 12 weeks of co-culture with MSC (n = 3) and MS-5 stromal cell line (n = 3). Each dot and circle represents one individual experiment, legend indicates the AML and MSC cells used. Error bar = SEM.
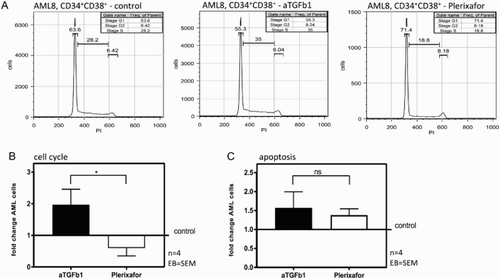
Blockade of TGF-β1 induces proliferation of AML cells while inhibition of CXCR4 is anti-proliferative
HSC-stromal interaction is regulated under physiologic conditions by cytokines, TGF-β1 and CXCL12 being two of the key factors [Citation5]. Since these signaling pathways are assumed to play an important role in aberrant hematopoiesis as well, we investigated the effect of blocking TGF-β1 and the CXCL12/CXCR4 axis on primary AML cell proliferation and cell cycle by flow cytometry using a CFSE-based proliferation assay and PI-based cell cycle analysis. (A) shows one representative FACS analysis of cell cycle progression of CD34+CD38+ AML cells (AML9). In both, the CD34+CD38+ and the CD34+CD38− AML subpopulations from two AML patients, inhibition of TGF-β1 was accompanied by significantly enhanced cell proliferation compared to control, whereas CXCR4 blockade significantly reduced the proportion of proliferating leukemic cells ((B); p = 0.008). This observation was confirmed by cell cycle analysis as shown exemplary in (A) (AML8). Congruent with the proliferation analysis, the number of AML cells in S- and G2-phase was significantly higher after TGF-β1 blockade, whereas inhibition of CXCR4 was associated with reduced AML cell proliferation ((B)). As shown in (C), there was no significant difference in induction of apoptosis by blocking TGF-β1 as compared to CXCR4.
Figure 3. Blockade of TGF-β1 enhanced the proliferation of AML cells while inhibition of CXCR4 showed anti-proliferative effects. (A) Representative zebra plot of CFSE stained CD34+CD38+ AML cells from one patient. (B) Absolute number of successive generations of CD34+CD38+ and CD34+CD38- AML cells (two patients: AML8 and AML9, four AML blast subpopulations), co-cultured with MSC + anti-TGF-β1 vs. MSC + plerixafor, relative to the results of the control dishes.
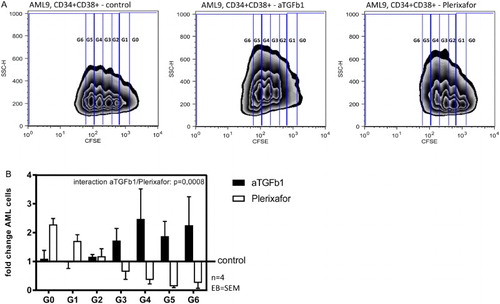
Figure 4. TGF-β1 blockade but not CXCR4 blockade results in cell cycle induction of AML blasts. (A) Representative histogram of PI stained CD34+CD38+ AML cells from one patient. Percentage of cells in S-, G1- and G2-phase are displayed. (B) S/G2-phase CD34+CD38+ and CD34+CD38- AML cells (two patients: AML8 and AML9, four AML blast subpopulations), co-cultured with MSC + anti-TGF-β1 vs. MSC + plerixafor, relative to the results of the control dishes. (C) Absolute count of apoptotic CD34+CD38+ and CD34+CD38− AML cells (two patients: AML8 and AML9, four AML blast subpopulations), co-cultured with MSC + anti-TGF-β1 vs. MSC + plerixafor, relative to the results of the control dishes.
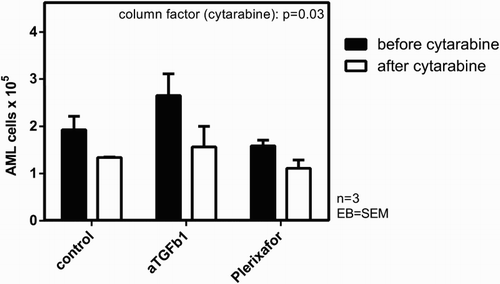
Combination of anti-TGF-β1 + cytarabine increased chemosensibility of AML cells, whereas plerixafor did not
Since we showed that blockade of TGF-β1 was associated with an increase in AML cell proliferation, we tested if this translates into an improved chemotherapy sensitivity by adding cytarabine to the co-culture. As shown in , inhibition of TGF-β1 induced a clear increase in cytarabine sensitivity compared to control. However, this difference did not reach statistical significance. In contrast to TGF-β1 inhibition, CXCR4 blockade did not result in enhanced chemosensibility to cytarabine in our experiments (AML samples 8–10).
Discussion
Human BM MSC support hematopoietic progenitor cells by preserving function and permitting proliferation, differentiation and mobilization. Currently, the question has emerged if BM stromal cells are also supportive to AML cells and for this reason favor chemotherapy resistance and cause leukemic relapse. Understanding stroma–leukemia interaction will help to understand the AML niche interaction biology. Therefore, we used MSC and AML cells co-culture as a model to mimic the in vivo environment within the BM of leukemia patients.
In order to generate an optimal in vitro co-culture model for testing agents disrupting the leukemia–stroma cell interaction, we first analyzed the difference between human primary MSC and the murine stromal cell line MS-5 in supporting the maintenance and growth of human primary AML cells. Recently, persistence of AML cells up to 6 weeks on MSC was described by the group of Larochelle [Citation16], while van Gosliga et al. showed long-term maintenance of leukemic blasts applying MS-5 cells [Citation15]. We demonstrated in a head to head comparison with primary cells that human MSCs sustain significantly more viable AML cells after 12-week long-term culture compared to MS-5 cells and therefore appear suitable to investigate the processes inside the leukemic niche. A MSC/MS-5 comparative study with similar results have recently been published by Antonelli et al., however following co-culture of two AML patients only for 30 days and without proving the required MSC ISCT criteria [Citation17]. As published by others, not all AML samples grow equally well under feeder cell conditions. For instance, AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1 hardly proliferate in vitro [Citation15]. By contrast, our AML5 sample (RUNX1-RUNX1T1 positive) showed the highest expansion capability of all samples in two individual co-culture experiments with different MSC populations and was twice as high as compared to MS-5 feeders ((D)).
TGF-β1 and CXCL12 are two of the key modulators of leukemia–stroma cell communications. In this work, we analyzed the functional role of TGF-β1 and the CXCL12/CXCR4 pathway within the leukemic niche and showed that the blockade of TGF-β1 increased AML cell proliferation, whereas the CXCR4 antagonist had an opposite effect. So far, only Tabe et al. investigated both pathways regarding leukemia–stroma cell interactions using primary AML cells as well as several leukemic cell lines [Citation12]. Nonetheless, they studied the effects on proliferation of primary AML samples solely by showing that the murine TGF-β1 antibody 1D11 can reverse human recombinant TGF-β1 (rh-TGF-β1) induced cell cycle arrest. Plerixafor alone or combinations of 1D11 and plerixafor did not influence cell cycle arrest in their MSC/AML cell co-culture system with the leukemic MV4-11 cell line. We on the other hand demonstrated considerable differences in primary AML cell proliferation in co-culture depending on either TGF-β1 or CXCL12/CXCR4 pathway inhibition. By CFSE assay, we could demonstrate an increase of AML cells in successive generations when TGF-β1 is inhibited, whereas plerixafor significantly reduced AML cell proliferation. Likewise, blockade of TGF-β1 by anti-TGF-β1 mAb induced an increase of AML cells in S/G2 cell cycle phase and reduced the cell proportion in G1 phase, corresponding to the data of Tabe et al. [Citation12]. By contrast, blockade of the CXCR4/CXCL12 axis showed here, too, an anti-proliferative effect. The anti-proliferative capacity of plerixafor was also observed by Tavor et al. [Citation18,Citation19] and others [Citation20,Citation21] which is in line with our findings. However, in contrast to the data of Tavor et al. [Citation19], our results showed fewer cells in S phase and an increase in G1 phase and thus enhanced cell cycle arrest. Despite significant differences regarding the proliferation of AML blasts in our in vitro co-culture model, it should be noted, that an extrapolation from in vitro experiments to the in vivo situation must be interpreted carefully.
To get further insights, we also investigated effects of anti-TGF-β1 and plerixafor on apoptosis. Tabe et al. proved increase of apoptotic MV4-11 cells by 1D11 only in the absence of MSC, confirming the significant protective effects of MSC on the survival of the leukemic cells [Citation12]. Agreeing with this, apoptosis of AML cells was not augmented after TGF-β1 blockade in our MSC co-culture system. Consistent with the mentioned ability to keep AML cells quiescent, CXCR4 inhibition did not influence cell apoptosis in our setting, as shown also by other groups [Citation10,Citation19,Citation20].
Several studies have indicated that by suppression of TGF-β1 or CXCR4, the ability of these cytokines to maintain hematopoietic/leukemic cells quiescent in G0/G1 phase is down-regulated. Thus, AML cells were unable to BM anchoring, were mobilized into peripheral blood and were more susceptible to the anti-leukemic effect of cytarabine [Citation7,Citation10,Citation12,Citation22]. This aspect was not investigated for both cytokines in vitro so far. We could demonstrate an increased chemosensibility by adding anti-TGF-β1, while AML cell loss did not change after application of plerixafor and cytarabine. These findings are in line with the data from our proliferation assays, which showed that anti-TGF-β1 and plerixafor act in contradictory manner. By augmenting the proliferation of AML cells, abrogation of TGF-β1 leads to increased chemosensibility. As described previously TGF-β1 transduces signals by binding to the serine/threonine kinase receptor TGF-β receptor II and thereby recruiting the type I receptor, followed by phosphorylation of Smad2/3 and translocation to the nucleus after complexing with Smad4, where transcriptional activation of target genes is regulated. Regarding TGF-β1 mediated growth inhibition and quiescence of hematopoietic cells, several mechanisms have been proposed, including alterations in cytokine receptor expression like c-kit or c-Mpl as well as upregulation of cyclin-dependent kinase inhibitors like p21 or p57. In addition, TGF-β1 was shown to induce an increased production of extracellular matrix components and integrins, which further facilitates the lodgment of hematopoietic stem cells as well as leukemic cells to the BM niche. Thus, inhibition of TGF-β1 prevents the above-mentioned effects and leads to an increased proliferation of leukemic cells, thereby making AML cells more susceptible for chemotherapeutic agents [Citation23–26].
Knowledge about the structure and function of the hematopoietic BM niche has markedly increased in the last years but still little is known about the processes within the leukemic niche. Our data demonstrate that human MSC are very potent feeder cells, able to maintain AML cells in long-term culture. This favorable co-existence seems to be due in part to key molecules important for intercellular communication within the niche. In our system, we show that TGF-β1 and CXCL12 are two of these major regulators. Blockade of these two pathways was associated with different effects on AML cell proliferation and chemotherapy resistance, probably complicated by autocrine signaling loops between the two pathways as described by Kojima et al. [Citation27].
Nonetheless, it should be mentioned that due to the small sample size and variable expansion of individual AML patient cells further experiments are needed to draw in-depth conclusions.
Target therapies including the CXCR4 antagonist plerixafor alone or in combination with chemotherapy or the TGF-β1 antagonist GC1008 in human cancer patients are currently tested in phase I–II clinical studies [Citation28,Citation29]. Combination of these agents with or without chemotherapy should be taken into consideration but will certainly benefit from increased knowledge about interaction modes between different pathways of niche networking.
Acknowledgments
We have had the support of Laboratories of José Carreras Center, University Hospital Regensburg for use of Becton Dickinson FACS ARIA IIu cell sorter. Therefore, we thank Monique Germerodt, Rüdiger Eder and Irina Fink. Besides, we thank Gero Brockhoff for reviewing the FACS data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014
- Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. doi: 10.1097/00007890-196803000-00009
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905
- Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262
- Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood. 2000;96(6):2022–2036.
- Ruscetti FW, Akel S, Bartelmez SH. Autocrine transforming growth factor-beta regulation of hematopoiesis: many outcomes that depend on the context. Oncogene. 2005;24(37):5751–5763. doi: 10.1038/sj.onc.1208921
- Xu Y, Tabe Y, Jin L, et al. TGF-beta receptor kinase inhibitor LY2109761 reverses the anti-apoptotic effects of TGF-beta1 in myelo-monocytic leukaemic cells co-cultured with stromal cells. Br J Haematol. 2008;142(2):192–201. doi: 10.1111/j.1365-2141.2008.07130.x
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807
- Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91(1):100–110.
- Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113(24):6215–6224. doi: 10.1182/blood-2008-05-158311
- Nervi B, Ramirez P, Retting MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123
- Tabe Y, Shi YX, Zeng Z, et al. TGF-beta-Neutralizing antibody 1D11 enhances cytarabine-induced apoptosis in AML cells in the bone marrow microenvironment. PLoS One. 2013;8(6):e62785. doi: 10.1371/journal.pone.0062785
- Buckley CD, Amft N, Bradfield PF, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165(6):3423–3429. doi: 10.4049/jimmunol.165.6.3423
- Katoh M. Integrative genomic analyses of CXCR4: transcriptional regulation of CXCR4 based on TGFbeta, nodal, activin signaling and POU5F1, FOXA2, FOXC2, FOXH1, SOX17, and GFI1 transcription factors. Int J Oncol. 2010;36(2):415–420.
- van Gosliga D, Schepers H, Rizo A, et al. Establishing long-term cultures with self-renewing acute myeloid leukemia stem/progenitor cells. Exp Hematol. 2007;35(10):1538–1549. doi: 10.1016/j.exphem.2007.07.001
- Ito S, Barrett AJ, Dutra A, et al. Long term maintenance of myeloid leukemic stem cells cultured with unrelated human mesenchymal stromal cells. Stem Cell Res. 2015;14(1):95–104. doi: 10.1016/j.scr.2014.11.007
- Antonelli A, Noort WA, Jaques J. Establishing human leukemia xenograft mouse models by implanting human bone marrow-like scaffold-based niches. Blood. 2016;128(25):2949–2959. doi: 10.1182/blood-2016-05-719021.
- Tavor S, Petit I, Porozov S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64(8):2817–2824. doi: 10.1158/0008-5472.CAN-03-3693
- Tavor S, Eisenbach M, Jacob-Hirsch J, et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia. 2008;22(12):2151–5158. doi: 10.1038/leu.2008.238
- Zhang Y, Patel S, Abdelouahab H, et al. CXCR4 inhibitors selectively eliminate CXCR4-expressing human acute myeloid leukemia cells in NOG mouse model. Cell Death Dis. 2012;3:e396. doi: 10.1038/cddis.2012.137
- Beider K, Begin M, Abraham M, et al. CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol. 2011;39(3):282–292. doi: 10.1016/j.exphem.2010.11.010
- Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119(17):3917–3924. doi: 10.1182/blood-2011-10-383406
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051
- Narayan S, Thangasamy T, Balusu R. Transforming growth factor-beta receptor signaling in cancer. Front Biosci. 2005;10:1135–1145. doi: 10.2741/1606
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033
- Blank U, Karlsson S. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia. 2011;25:1379–1388. doi: 10.1038/leu.2011.95
- Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–20014. doi: 10.1073/pnas.1013805107
- Morris JC, Shapiro GI, Tan AR, et al. Phase I/II study of GC1008: A human anti-transforming growth factor-beta (TGFb) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC). J Clin Oncol. 2008;26, abstr. 9028.
- Peled A, Tavor S. Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics. 2013;3(1):34–39. doi: 10.7150/thno.5150